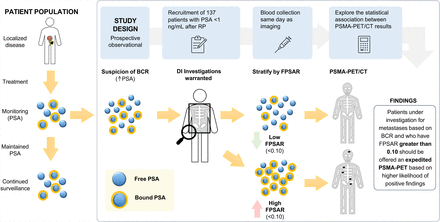
Visual Abstract
Abstract
In Canada and across the globe, access to PSMA PET/CT is limited and expensive. For patients with biochemical recurrence (BCR) after treatment for prostate cancer, novel strategies are needed to better stratify patients who may or may not benefit from a PSMA PET scan. The role of the free-to-total prostate-specific antigen (PSA) ratio (FPSAR) in posttreatment prostate cancer, specifically in the PSMA PET/CT era, remains unknown. Our aim in this study was to determine the association of FPSAR in patients referred for 18F-DCFPyL PSMA PET/CT in the BCR setting and assess the correlation between FPSAR and 18F-DCFPyL PSMA PET/CT positivity (local recurrence or distant metastases). Methods: This prospective study included 137 patients who were referred for 18F-DCFPyL PSMA PET/CT and had BCR with a total PSA of less than 1 ng/mL after radical prostatectomy (RP) (including adjuvant or salvage radiotherapy). Blood samples were collected on the day of 18F-DCFPyL PSMA PET/CT. FPSAR was categorized as less than 0.10 or as 0.10 or more. A positive 18F-DCFPyL PSMA PET/CT scan was defined by a PROMISE classification lesion score of 2 or 3, irrespective of the site of increased tracer uptake (e.g., prostate, pelvic nodes, bone, or viscera). Results: Overall, 137 blood samples of patients with BCR after RP were analyzed to calculate FPSAR. The median age at 18F-DCFPyL PSMA PET/CT was 68.6 y (interquartile range, 63.0–72.4 y), and the median PSA at 18F-DCFPyL PSMA PET/CT was 0.3 ng/mL (interquartile range, 0.3–0.6 ng/mL). Eighty-six patients (62.8%) had an FPSAR of less than 0.10, whereas 51 patients (37.2%) had an FPSAR of 0.10 or more. An FPSAR of 0.10 or more was identified as an independent predictor of a positive 18F-DCFPyL PSMA PET/CT scan, with an odds ratio of 6.99 (95% CI, 2.96–16.51; P < 0.001). Conclusion: An FPSAR of 0.10 or more after RP independently correlated with increased odds of a positive 18F-DCFPyL PSMA PET/CT scan among BCR post-RP patients. These findings may offer an inexpensive method by which to triage access to 18F-DCFPyL PSMA PET/CT in jurisdictions where availability is not replete.
Prostate-specific antigen (PSA) is a protein made predominantly in the prostate gland and is a widely used biomarker for the detection, treatment, and prognostication of patients with prostate cancer (PCa). PSA exists in 6 isoforms in the blood; the majority are bound to plasma proteins, however, 1 form is unbound and floats freely in the serum, called free PSA (1). The free-to-total PSA ratio (FPSAR) is the ratio of free circulating PSA to the total PSA level. FPSAR has been used in tandem with the total PSA level to predict the probability of PCa in the screening or diagnostic setting.
Historically, FPSAR was used in the ambiguous total PSA range of 4–10 ng/mL, with a higher FPSAR (>0.25) incurring a lower chance of PCa diagnosis and a lower Gleason sum (2). On the basis of these findings, FPSAR has been proposed as a tool for differentiating between PCa and benign prostatic hyperplasia in patients with intermediate levels of PSA (4–10 ng/mL) (2,3).
Our group has recently become interested in the role of FPSAR as a biomarker among patients who have recurrent PCa, as we have noticed that patients with high free PSA, unlike in the diagnostic setting, tend to have poorer outcomes. In a recent analysis, we assessed the utility of FPSAR as a biomarker of disease recurrence after definitive therapy. In contrast to the diagnostic setting, an FPSAR of 0.10 or more after treatment was associated with a higher risk of recurrence and more aggressive disease. This observation was validated in 3 separate cohorts. These cohorts included men who failed treatment with surgery, surgery plus radiotherapy, and primary radiotherapy (4,5). A particularly important observation was that a high FPSAR was also associated with distant metastatic disease recurrence.
The detection of locally recurrent or metastatic PCa has been revolutionized by the emergence of prostate-specific membrane antigen (PSMA) PET imaging because of its higher detection rates of recurrent or metastatic disease (6). The detection rate of local recurrence or distant metastases is approximately 70% in patients with a serum PSA concentration higher than 1 ng/mL (7).
Our aim in this study was to assess the correlation between FPSAR and 18F-DCFPyL PSMA PET/CT positivity (local recurrence or distant metastases) in patients referred for 18F-DCFPyL PSMA PET/CT in the biochemical recurrence (BCR) setting and assess the correlation between FPSAR and 18F-DCFPyL PSMA PET/CT positivity (local recurrence or distant metastases). Additionally, we assessed the correlation between FPSAR and other 18F-DCFPyL PSMA PET/CT findings.
MATERIALS AND METHODS
Study Design and Setting
This study was a single-center, prospective trial designed to test the hypothesis that a high FPSAR (≥0.10) in patients with BCR after radical prostatectomy (RP) would be associated with a positive 18F-DCFPyL PSMA PET/CT scan. The study was conducted at the Princess Margaret Cancer Centre, and recruitment began on February 25, 2022, with the last patient enrolled on August 11, 2023. Patients referred for 18F-DCFPyL PSMA PET/CT who experienced BCR and had a total PSA level below 1 ng/mL after undergoing RP (including adjuvant or salvage radiotherapy) were invited to participate in the study. Of note, all patients had an undetectable post-RP PSA. The study protocol is available as Supplemental Appendix 1 (supplemental materials are available at http://jnm.snmjournals.org) (8,9).
A positive 18F-DCFPyL PSMA PET/CT scan was defined by a PROMISE classification lesion score of 2 or 3 (10), irrespective of the site of increased tracer uptake (e.g., prostate, pelvic nodes, bone, or viscera). Focal radiotracer uptake was graded according to the degree of radiotracer uptake, compared with reference tissues. All 18F-DCFPyL PSMA PET/CT scans were interpreted by specialist genitourinary radiologists or nuclear medicine physicians, and the PSMA ligand used for the examination was 18F-DCFPyL (also known as 18F-piflufolastat or Pylarify [Lantheus]).
A blood sample (1 serum-separating tube containing approximately 5 mL) was collected on the day of the 18F-DCFPyL PSMA PET/CT scan. Each blood sample underwent analysis for total PSA and free PSA (as per our prior methodology (5)). The ratio (free to total PSA) was derived from these results. Institutional Research Ethics Board approval was obtained for this study (REB 21-5555), and all subjects gave written informed consent.
Study Population
In total, 137 patients experiencing BCR after RP or adjuvant/salvage radiotherapy for PCa were included in the study. Four patients of these 137 underwent 18F-DCFPyL PSMA PET/CT before RP. The eligibility criteria for participation encompassed individuals aged 18 y or older who were capable of providing informed consent and had previously undergone surgical treatment for PCa (some additionally receiving adjuvant or salvage radiotherapy). Participants were required to be scheduled for a 18F-DCFPyL PSMA PET/CT scan and to meet the criteria for BCR, defined as having a postoperative PSA value exceeding 0.1 ng/mL or—for those treated with adjuvant/salvage radiotherapy—exceeding 0.2 ng/mL, while maintaining a total PSA level below 1 ng/mL. The exclusion criteria comprised PCa patients currently undergoing hormonal therapy or those who had received hormonal therapy within the past year, as well as individuals with a history of primary radiation therapy for PCa.
Study Outcomes and Variables
The primary outcome was to evaluate the correlation between FPSAR and positivity (local recurrence or distant metastasis) on 18F-DCFPyL PSMA PET/CT, aiming to understand the role that FPSAR plays in disease recurrence.
Patients were categorized into one of the following groups, based on the presence and type of 18F-DCFPyL PSMA PET/CT uptake observed: no metastases, bone metastases, lymphatic metastases, prostatic-fossa–only uptake, visceral metastases (liver and lung), 18F-DCFPyL PSMA PET/CT–positive (any site positive), and 18F-DCFPyL PSMA PET/CT–negative (all sites negative). Of note, patients with local recurrence in the prostatic fossa were not considered metastatic. For modeling purposes, patients were also categorized using 1 of 4 dichotomizations: presence of any metastases (i.e., lymphatic, bone, or visceral) versus no metastases, presence of lymphatic metastases versus no metastases, presence of lymphatic metastases/bone metastases versus no metastases, and 18F-DCFPyL PSMA PET/CT–positive versus 18F-DCFPyL PSMA PET/CT–negative.
Clinical data were gathered from electronic records, encompassing information such as age; race/ethnicity; administration of hormonal therapy, adjuvant radiotherapy, or salvage radiotherapy; PSA levels at both diagnosis and BCR; pathologic findings from RP and biopsy; and results of 18F-DCFPyL PSMA PET/CT.
Statistical Analysis
Descriptive statistics were used to characterize patient characteristics, clinical characteristics, and outcome distribution, and the results were stratified by low versus high FPSAR. On the basis of our prior analyses, an FPSAR cut point of 0.10 was chosen (5). Depending on the expected outcome frequencies, the χ2 test or Fisher exact test was used to assess differences in outcome distributions across groups. For each outcome, univariable logistic regression models incorporating FPSAR (<0.10 vs. ≥0.10), Gleason grade group (GG) (GG 1–3 vs. GG 4–5), total PSA (0.10–0.49 vs. 0.50–1 ng/mL), time from BCR to 18F-DCFPyL PSMA PET/CT, pT stage (T2 vs. T3a vs. T3b), and positive margin and lymph node status (negative vs. positive vs. not performed) as predictors were fit. Predictors with significant P values in the univariable analysis were incorporated into a multivariable logistic regression model. As an exploratory analysis, a univariable logistic regression model using FPSAR as a continuous predictor of the primary outcome was fit, and the resulting predicted probability of positive 18F-DCFPyL PSMA PET/CT by FPSAR was visualized.
Additionally, patients were categorized by total PSA (<0.2, 0.2–0.49, and 0.50–0.99 ng/mL) and by both GG (GG 1–3 and GG 4–5) and FPSAR (<0.10 and ≥0.10). Differences in outcome distribution by total PSA category and by GG/FPSAR category were assessed using χ2 or Fisher exact tests. Statistical analyses were conducted using R version 4.3.0 (R Core Team). All hypothesis tests were 2-sided, and P values of less than 0.05 were considered statistically significant.
RESULTS
Overall Cohort Characteristics
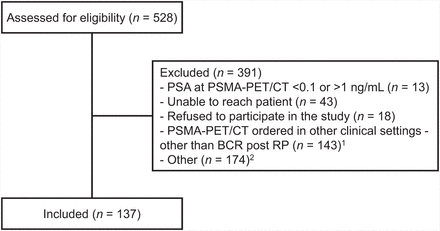
To compute FPSAR, 137 blood samples from patients experiencing BCR after RP were analyzed. The flow of participants throughout the study is depicted in Figure 1. The median age at 18F-DCFPyL PSMA PET/CT was 68.6 y (interquartile range, 63.0–72.4 y), and the median PSA at 18F-DCFPyL PSMA PET/CT was 0.3 ng/mL (interquartile range, 0.3–0.6 ng/mL). The GG distribution at RP was as follows: GG 1 in 6 patients (4.6%), GG 2 in 69 (53.1%), GG 3 in 35 (26.9%), GG 4 in 6 (4.6%), and GG 5 in 14 (10.8%). The median time from BCR to 18F-DCFPyL PSMA PET/CT was 7.8 mo (interquartile range, 1.8–21.0 mo). Regarding T stage, margins, and lymph nodes, 43 patients (35.2%) were pT2 at final RP, whereas 79 (64.8%) were pT3 or higher; 57 patients (42.9%) had positive margins; and 15 (10.9%) had positive lymph nodes (Table 1).
Study flowchart. 1Patients with history of primary radiation therapy for PCa, those currently receiving hormonal therapy, and PCa patients who have received hormonal therapy within past year (outside of neoadjuvant setting). 2Included scan and clinic cancellations, patient no-shows, same-day refusals for scans or to provide blood samples, language barriers, and patients missed by contact.
Patient Characteristics Overall and Stratified by FPSAR < 0.10 and FPSAR ≥ 0.10
Summary of 18F-DCFPyL PSMA PET/CT Outcomes
Table 2 shows the pattern of metastases in RP patients undergoing 18F-DCFPyL PSMA PET/CT for BCR. 18F-DCFPyL PSMA PET/CT was negative in 67 patients (48.9%) and positive in 70 patients (51.1%).
Patterns of Metastases in RP Patients Undergoing PSMA PET/CT for Biochemical Failure (n = 137)
Fifty-one (37.2%) patients were metastatic only (without prostatic fossa involvement). Nine patients (6.6%) had prostate fossa involvement only. Ten patients (7.3%) had both metastatic and prostate fossa involvement. Among the metastatic patients, bone metastases were observed in 16 (26.2%), with 7 (11.5%) exclusively affecting bone; lymphatic metastases were present in 52 (85.2%), with 40 (65.5%) exclusively involving lymph nodes. One (1.6%) patient had liver metastases, and 3 patients (4.9%) had lung metastases, with 1 (1.6%) of these patients having lung metastases only.
FPSAR and Total PSA in Relation to Metastatic Status
Table 3 shows FPSAR in relation to 18F-DCFPyL PSMA PET/CT metastatic status. Eighty-six patients (62.8%) had an FPSAR of less than 0.10, whereas 51 patients (37.2%) had an FPSAR of 0.10 or more. Baseline patient characteristics categorized by FPSAR group are detailed in Table 1. Forty (57.1%) patients with a positive 18F-DCFPyL PSMA PET/CT scan had an FPSAR of 0.10 or more, whereas 30 (42.9%) had an FPSAR of less than 0.10. In contrast, among patients with a negative 18F-DCFPyL PSMA PET/CT scan, 11 (16.4%) had an FPSAR of 0.10 or more and 56 (83.6%) had an FPSAR of less than 0.10 (P < 0.001).
Cross Tabulation for Metastasis Status According to FPSAR
When comparing patients who had any metastases (i.e., bone, lymph nodes, liver, or lungs) with patients who had no metastases, we found that 35 (57.4%) exhibited an FPSAR of 0.10 or more and 26 (42.6%) an FPSAR of less than 0.10 in the former group, whereas 16 (21.1%) demonstrated an FPSAR of 0.10 or more and 60 (78.9%) an FPSAR of less than 0.10 in the latter group (P < 0.001). In comparing patients who had lymphatic metastases with those who had none, we found that 32 (61.5%) showed an FPSAR of 0.10 or more and 20 (38.5%) an FPSAR of less than 0.10 in the former group, whereas 20 (38.5%) demonstrated an FPSAR of 0.10 or more and 60 (78.9%) an FPSAR of less than 0.10 in the latter group (P < 0.001).
No difference was observed in FPSAR among patients with only prostatic fossa recurrence versus those with any metastases. Similarly, there was no difference in FPSAR between patients with lymphatic metastases and those with bone metastases or between those with no metastases and those with bone metastases.
Supplemental Table 1 presents total PSA in relation to 18F-DCFPyL PSMA PET/CT metastatic status. There was no difference in total PSA across the PSA categories of 0–0.19, 0.2–0.49, and 0.5–0.99 ng/mL and the main outcomes of 18F-DCFPyL PSMA PET/CT.
Predictors of 18F-DCFPyL PSMA PET/CT Findings
Univariable and multivariable logistic regression models are presented in Table 4. First, in the comparison between 18F-DCFPyL PSMA PET/CT–positive and 18F-DCFPyL PSMA PET/CT–negative groups, both GG 4–5 at RP and an FPSAR of 0.10 or more were identified as independent predictors of a positive scan, with odds ratios (ORs) of 6.89 (95% CI, 1.78–26.67; P = 0.005) and 6.99 (95% CI, 2.96–16.51; P < 0.001), respectively. When any metastases versus no metastases were compared in the multivariate analysis, 2 factors emerged as independent predictors: GG 4–5 at RP (OR, 6.43; 95% CI, 1.90–21.82; P = 0.003) and an FPSAR of 0.10 or more (OR, 5.00; 95% CI, 2.24–11.17; P < 0.001). Similarly, when lymphatic metastases versus no metastases were compared, 2 factors also emerged as independent predictors: GG 4–5 at RP (OR, 10.53; 95% CI, 2.45–45.20; P = 0.002) and an FPSAR of 0.10 or more (OR, 6.39; 95% CI, 2.49–16.36; P < 0.001). Furthermore, when lymphatic and bone metastases versus no metastases were compared, again GG 4–5 at RP (OR, 5.06; 95% CI, 2.24–11.42; P < 0.001) and an FPSAR of 0.10 or more (OR, 6.78; 95% CI, 1.99–23.09; P = 0.002) were identified as independent predictors (Table 4).
Univariate and Multivariate Regression Models for Predictors by Metastasis Status
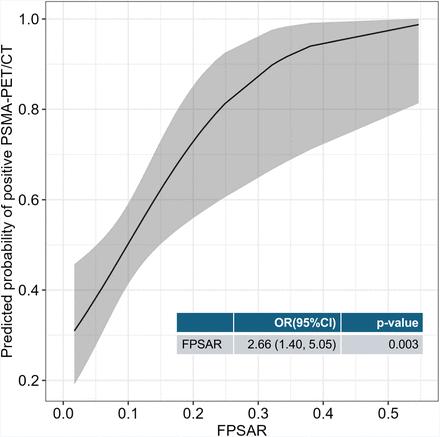
When modeling FPSAR as a continuous predictor, we found FPSAR to be positively associated with a positive 18F-DCFPyL PSMA PET/CT scan (OR, 2.66; 95% CI, 1.40–5.05; P = 0.003). The predicted probability of a positive 18F-DCFPyL PSMA PET/CT scan by FPSAR is visualized in Figure 2.
Univariate logistic regression models predicting odds of positive 18F-DCFPyL PSMA PET/CT using FPSAR (continuous) as predictor. To ease interpretation, FPSAR was multiplied by 10, and therefore OR can be interpreted as increase in odds per 0.1-unit increase in FPSAR.
FPSAR in Relation to RP GG and Metastatic Status
We attempted to identify a low-risk group of patients based on GG 1–3 disease at RP and an FPSAR of less than 0.10 and a high risk group based on GG 4–5 at RP and an FPSAR of 0.10 or more (Table 5). Among patients with an FPSAR of 0.10 or more and GG 4–5, 9 individuals (90.0%) exhibited a positive 18F-DCFPyL PSMA PET/CT scan, whereas 1 (10.0%) demonstrated a negative 18F-DCFPyL PSMA PET/CT scan (P < 0.001). When patients with any metastases versus no metastases were compared within the high-risk group, 8 patients (80.0%) had any metastases and 2 patients (20.0%) had no metastases. Conversely, among patients with an FPSAR of less than 0.10 and GG 1–3, 17 patients (23.6%) had any metastases and 55 (76.4%) no metastases.
Outcomes by FPSAR/GG Category
DISCUSSION
The FPSAR has shown clinical utility in distinguishing elevated PSA due to PCa from benign prostatic hyperplasia in the screening setting. It is often noted that patients with PCa typically exhibit a lower FPSAR (11). However, in the BCR setting, the converse has been consistently observed.
Three small-scale studies have previously interrogated the significance of FPSAR after RP, involving 38 (11), 46 (12), and 52 (13) patients. Across all 3 studies, a consistent finding emerged: roughly a fifth of the patients exhibited an FPSAR exceeding 0.17. Moreover, Wojno et al. (14) proposed a correlation between a posttreatment FPSAR surpassing 0.15 and aggressive disease characteristics in the pathology specimen. More recently, 2 studies from our group found an association between FPSAR and more aggressive disease. First, Goldberg et al. (4) demonstrated that patients with an FPSAR of 0.10 or more were more likely to have progression to castration-resistant PCa and metastasis. The authors also demonstrated that patients who underwent curative-intent external-beam radiation therapy for PCa showed higher biopsy Gleason scores among patients with an FPSAR of 0.10 or more. Multivariable analyses also showed that an FPSAR of 0.10 or more was associated with increased metastasis in the RP cohort (hazard ratio, 1.915; 95% CI, 1.241–2.955) and radiation therapy cohort (hazard ratio, 1.754; 95% CI, 1.112–2.769) and increased castration-resistant PCa in the RP cohort (hazard ratio, 2.470; 95% CI, 1.493–4.088). Second, Woon et al. (5) concluded that patients with an FPSAR of 0.15 or greater were started on ADT earlier, and they had progression to castration-resistant PCa and metastatic stage earlier.
However, these studies did not evaluate the relationship between FPSAR and detected metastases using imaging methods such as PSMA PET/CT. To our knowledge, the present study was the first to characterize the association of FPSAR at the time of BCR after RP and its potential as a predictive biomarker for PSMA PET/CT–detected metastases.
Stricker et al. assessed 164 patients who underwent imaging with PSMA PET/CT for a rising PSA level after RP with PSA levels of less than 0.5 ng/mL. In patients with a negative PSMA PET/CT scan who received salvage radiotherapy, 85% (23/27) demonstrated a treatment response, compared with a further PSA increase in 65% of those not treated (22/34). In the 36 of 99 patients with disease confined to the prostate fossa on PSMA PET, 83% (29/36) responded to salvage radiation (12). Thus, PSMA PET scans might stratify men into a group with a high response to salvage radiotherapy (negative findings or recurrence confined to the prostate) and a group with a poor response (positive nodes or distant disease). Although PSMA PET/CT is recognized for its high accuracy in detecting metastasis, its widespread use is hindered by cost constraints. In a metaanalysis published by Perera et al., which included 4,790 patients across PSA categories of 0–0.19, 0.2–0.49, 0.5–0.99, 1–1.99, and 2 ng/mL, the percentages of positive scans were 33%, 46%, 57%, 82%, and 97%, respectively (13). It should be noted that there was no measurable association between total PSA levels and 18F-DCFPyL PSMA PET/CT findings in our study (Supplemental Table 1). This likely reflects a narrow range of PSA values based on our inclusion criteria of a clinical PSA of 0.1–1 ng/mL.
Hence, the identification of predictors for metastatic disease holds significant clinical value. It aids in the improved stratification of patients who experience BCR after RP and assists in prioritizing 18F-DCFPyL PSMA PET/CT scheduling. Patients with an FPSAR of 0.10 or more should be prioritized.
In our prospective study involving 137 patients with BCR after RP, we established that an FPSAR of 0.10 or more and GG 4 or GG 5 pathology at RP were independently associated with a 5.00- and 6.43-fold increased likelihood of metastatic disease, respectively. Similarly, an FPSAR of 0.10 or more and GG 4 and GG 5 pathology at RP were also independently associated with a 6.39- and 10.53-fold increased likelihood of lymphatic metastases, respectively, when compared with patients with no metastases.
Furthermore, in the comparison between patients with lymphatic and bone metastases, all patients with lymphatic metastases exhibited an FPSAR of 0.10 or more and GG 4–5 disease. Likewise, when patients who had lymphatic metastases were compared with those who had other metastases, all patients with lymphatic metastases demonstrated an FPSAR of 0.10 or more and GG 4–5. These findings suggest a lymphotropic predilection with an FPSAR of 0.10 or more among patients with GG 4–5 at final pathology. We acknowledge that the number of patients in this subset is small; however, the finding remains an important one that warrants validation in larger cohorts. In a recent paper (15), our group found that the presence of a cribriform pattern at RP among patients with recurrence was linked to specific patterns of metastatic spread, resulting in a 3.13- and 2.08-fold increased likelihood of developing lymphatic metastases compared with bone or visceral metastases. In the pretreatment setting, we found intraductal carcinoma at biopsy as a predictor of lymphatic metastases (16). In the current project, we were unable to establish a correlation between these 2 features: FPSAR and patterns of spread. This limitation arose primarily because a significant portion of the specimens lacked annotation regarding these patterns. This was either due to RP’s being conducted outside our center or at a time when reporting on these patterns was not yet routine (i.e., before 2015).
The exact biologic mechanisms underlying this high-FPSAR phenomenon among recurrent PCa patients remain unclear. We propose that variations in PSA processing occur before RP. Essentially, after the removal of the prostate gland, cells producing PSA at metastatic sites release a less complexed form of PSA or, conversely, secrete higher levels of free PSA into the circulation. Consequently, an elevated FPSAR in individuals with detectable PSA after RP, subsequent to a period of undetectable PSA, suggests that malignant PCa cells at metastatic sites are the likely source of PSA, rather than residual benign prostate cells (4).
This study validated our previous retrospective studies confirming that a higher FPSAR confers an increased risk of metastases, which is the opposite of the case in the screening or diagnostic setting. Given that patients with a high FPSAR have a higher rate of metastatic disease on 18F-DCFPyL PSMA PET/CT, this could potentially be used to guide the frequency of follow-up and imaging to assess for metastatic disease. The result would be an improvement in the cost-effectiveness of imaging strategies and, ultimately, in patient outcomes.
A recent systematic review published by Pozdnyakov et al. (17) investigated PSMA PET/CT in patients with BCR after primary therapy for PCa. The authors reported that PSMA PET/CT yielded positive results in over two thirds of patients (68.2%). Additionally, they identified several factors associated with a positive PSMA PET/CT scan, including a Gleason score of at least 8 and higher serum PSA levels both at the initial diagnosis and at the time of PSMA PET/CT. The high sensitivity of PSMA PET/CT in detecting sites of disease recurrence led to frequent changes in management, reported in more than half of individuals after PSMA PET/CT. PSMA PET–directed management resulted in a biochemical response in most patients (72.4%; range, 21%–94%). Therefore, it is important to develop strategies to predict positive PSMA PET/CT scans so that patient selection can optimize subsequent treatment steps. We found that GG 4–5 and an FPSAR of 0.10 or more were independently associated with a 6.89- and 6.99-fold increase, respectively, in the odds of a positive 18F-DCFPyL PSMA PET/CT scan.
Our present study had several limitations. Although the overall number of subjects was sufficient, the subgroup of subjects with any form of metastases, including bone and visceral metastases, was relatively small. Consequently, this limitation led to notably wide 95% CIs in certain instances and precluded the feasibility of conducting multivariable regression analysis for specific metastatic outcomes. Additionally, the study was conducted at a single center, and long-term oncologic outcome data for this cohort of patients are unavailable. However, it is worth noting that the samples were prospectively collected, and FPSAR was performed by the same laboratory in a systematic and standardized manner.
CONCLUSION
An FPSAR of 0.10 or more after RP was independently associated with increased odds of a positive 18F-DCFPyL PSMA PET/CT scan among BCR post-RP patients. These findings may provide a cost-effective method to prioritize access to PSMA PET/CT in regions with limited availability.
DISCLOSURE
Rui Bernardino is supported by FCT (2022.13386.BD). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is FPSAR associated with 18F-DCFPyL PSMA PET/CT findings in patients experiencing BCR after RP?
PERTINENT FINDINGS: In this prospective study of 137 patients referred for PSMA PET/CT after RP and BCR with a total PSA of less than 1 ng/mL, an FPSAR of 0.10 or more was independently associated with a 6.99-fold increase in the odds of a positive 18F-DCFPyL PSMA PET/CT scan.
IMPLICATIONS FOR PATIENT CARE: These results suggest that FPSAR could serve as a cost-effective tool to prioritize access to PSMA PET/CT in regions with limited availability.
Footnotes
Published online Sep. 26, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication April 1, 2024.
- Accepted for publication August 28, 2024.