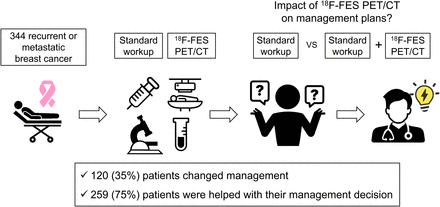
Visual Abstract
Abstract
The clinical impact of 16α-18F-fluoro-17β-estradiol (18F-FES) PET/CT on patient management has not been well investigated. The aim of this study was to assess the clinical impact of 18F-FES PET/CT on the management of patients with recurrent or metastatic breast cancer. Methods: Study subjects were identified retrospectively from a database of a prospective trial for postmarketing surveillance of 18F-FES between 2021 and 2023. Patients who were suspected or known to have recurrent or metastatic estrogen receptor–positive breast cancer based on a routine standard workup were included. Planned management before and actual management after 18F-FES PET/CT were assessed by 2 experienced medical oncologists via medical chart review. A 5-point questionnaire was provided to evaluate the value of 18F-FES PET/CT for management planning. The rate of intention-to-treat and interdisciplinary changes, and the impact of 18F-FES PET/CT according to PET/CT result or clinical indication, were examined. Results: Of the 344 included patients, 120 (35%) experienced a change in management after 18F-FES PET/CT. In 139 (40%) patients,18F-FES PET/CT supported the existing management decision without a change in management. Intention-to-treat and interdisciplinary changes accounted for 64% (77/120) and 68% (82/120) of all changes, respectively. A higher rate of change was observed when lesions were 18F-FES–negative (44% [36/81]) than 18F-FES–positive (30% [51/172]) or mixed 18F-FES–positive/negative (36% [33/91]). Regarding clinical indications, the highest rate of change was shown when evaluating the origins of metastasis of double primary cancers (64% [9/14]). Conclusion: 18F-FES PET/CT modified the management of recurrent or metastatic breast cancer, serving as an impactful imaging modality in clinical practice.
Breast cancer is the most diagnosed cancer worldwide, and it is estimated that 30% of all new cases of cancer in women in 2024 in the United States will be breast cancer (1). Despite favorable outcomes for operable breast cancer, the 5-y survival rate of de novo metastatic breast cancer, accounting for 3%–6% of newly diagnosed breast cancer, is only 31% (2). Furthermore, 15%–20% of patients with early breast cancer experience a recurrence within 10 y (3). Estrogen receptor (ER) is expressed by 80% of breast cancer (4) and is used as a target for treatment and regarded as a crucial predictive and prognostic marker. ER status is typically determined through immunohistochemistry, serving as the reference standard (5); however, biopsy is an invasive procedure, may result in sampling errors, and may not be feasible for all lesions. Additionally, spatial and temporal heterogeneity of ER expression within the same patient presents a clinical challenge, and the discordance rate between the primary tumor and metastasis is as high as 30%–40% (6).
16α-18F-fluoro-17β-estradiol (18F-FES) binds to ER in a manner similar to that of estradiol and allows for in vivo PET/CT imaging of ER expression by recurrent or metastatic tumors throughout the whole body, enabling assessment of ER status in hard-to-biopsy locations and the heterogeneity of ER expression (7). A high concordance has been observed between 18F-FES uptake on imaging and immunohistochemistry results (8,9). 18F-FES PET/CT has been reported to improve clinicians’ diagnostic understanding (10), influence therapeutic decisions (10,11), and resolve clinical dilemmas in breast cancer patients (10,12). Recently, appropriate-use criteria for 18F-FES PET/CT were published by the expert group of the Society of Nuclear Medicine and Molecular Imaging to promote its clinical use (13). However, comprehensive analysis of the impact of 18F-FES PET/CT on patient management in routine practice, its clinical significance, and the factors contributing to its impact is still lacking. Therefore, using a postmarketing surveillance database, we sought to evaluate the impact of 18F-FES PET/CT on clinical decisions in the management of patients with recurrent or metastatic breast cancer.
MATERIALS AND METHODS
Study Design
The study patients were identified from the database of a prospective trial for postmarketing surveillance of 18F-FES conducted between April 2021 and April 2023 at the Asan Medical Center. Details of the postmarketing surveillance trial are provided in the supplemental materials (supplemental materials are available at http://jnm.snmjournals.org).
A consecutive series of patients from the postmarketing surveillance database were included in the study. Patients were excluded if their primary breast cancer was ER-negative or if there was an immediate loss to follow-up, preventing assessment of the implemented management after 18F-FES PET/CT. Our institutional review board approved the study protocol (2023-1518) and waived the requirement for informed consent for this retrospective analysis.
The routine standard workup adhered to the National Comprehensive Cancer Network guideline (14) as described in the supplemental materials. Data were collected on all standard workups within a maximum interval of 3 mo between the standard workup and 18F-FES PET/CT.
18F-FES PET/CT Imaging and Interpretation
18F-FES was prepared as previously described (15). 18F-FES PET/CT images were obtained using a PET/CT scanner (Biograph Vision 600; Siemens Healthineers) 90 ± 10 min after intravenous administration of a dose of 111–222 MBq of 18F-FES (8,16). Details on the 18F-FES PET/CT acquisition are provided in the supplemental materials.
Images were prospectively assessed at the time of acquisition by 2 independent board-certified nuclear medicine physicians with 11 and 31 y of experience, who had access to other clinical information. Lesions with 18F-FES uptake higher than the local background activity were visually interpreted as 18F-FES–positive, whereas lesions identified on standard workup or combined low-dose CT that showed intensity equivocal to or less than the background activity were interpreted as 18F-FES–negative (8,16).
Outcomes
The primary outcome of the study was to assess the impact of 18F-FES PET/CT on management in terms of the percentage of cases in which the clinician’s management plans were changed according to the 18F-FES PET/CT findings in comparison with standard workup. Secondary outcome measures were to assess the proportion of intention-to-treat and interdisciplinary changes in management, the impact of 18F-FES PET/CT on management according to the results of 18F-FES PET/CT (positive, negative, or mixed), and the impact of 18F-FES PET/CT on management according to the different clinical indications. Intention-to-treat change was defined as alteration of the treatment intention (i.e., from a curative to palliative approach), and interdisciplinary change was defined as modification of the type of management (i.e., surgery, endocrine treatment, chemotherapy, radiotherapy, or multidisciplinary treatment) (17). For 18F-FES–negative lesions, whether the lesions were malignant or benign was determined either by biopsy or through clinical judgment based on medical history and standard imaging workup. The clinical indications for referring a patient for 18F-FES PET/CT were categorized according to the appropriate-use criteria (13). In addition, a case of double primary cancer with an unclear origin of the metastatic lesion was classified into a separate clinical indication, as proposed by a previous study (12).
The 18F-FES PET/CT and clinical records of patients, including the planned management before 18F-FES PET/CT and the actual management after 18F-FES PET/CT, as recorded on medical charts by the multidisciplinary team (including nuclear medicine physicians, breast surgeons, medical oncologists, radiation oncologists, radiologists, and pathologists) or referring physicians, were independently reviewed by 2 medical oncologists specialized in breast cancer, with 8 and 17 y of experience. Additionally, a 5-point questionnaire modified from a previous study (10) was provided to categorize the impact of 18F-FES PET/CT on management change (Table 1), with any discrepancy being resolved via discussion. We judged that 18F-FES PET/CT changed the patient management if the result of the questionnaire was category 1, 4, or 5 and that 18F-FES PET/CT was helpful in the clinical decision if the category was 3, 4, or 5.
Questionnaire for Clinicians for Determining Impact of 18F-FES PET/CT on Management Plans
Statistical Analysis
Descriptive statistics were used to determine the impact of 18F-FES PET/CT on management change. Continuous variables are expressed as mean ± SD or median and interquartile range. Categoric variables are expressed as number and percentage. Sankey diagrams visualizing the change in management were created using the ChartExpo plug-in for Microsoft Excel (PolyVista Inc.). Proportions of management change are presented with 95% Clopper–Pearson CIs and compared using χ2 tests. All statistical analyses were performed using R software (version 4.1.4; R Foundation for Statistical Computing).
RESULTS
Patient Characteristics
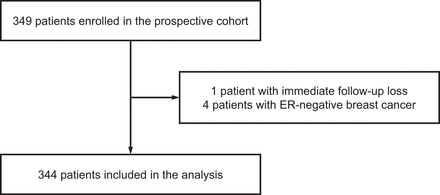
Of 349 patients enrolled in the original prospective study, 344 were included in this analysis (Fig. 1). The patient characteristics are summarized in Table 2. The histopathology results of the primary tumors were invasive ductal carcinoma in 287 (83%) patients and invasive lobular carcinoma in 36 (11%). Of note, among these 339 patients, 3 were diagnosed with bilateral breast cancer and one had 2 malignant tumors in the right breast, with one being both ER- and progesterone receptor–positive, and the other being negative for both. Progesterone receptor and human epidermal growth factor receptor 2 expression of the primary tumor was positive in 241 (70%) and 33 (10%) patients, respectively. A total of 170 (49%) patients underwent biopsy at the time of 18F-FES PET/CT, 93 of whom underwent biopsy for suspected recurrent or metastatic lesions. Of these 93 patients, 67 underwent immunohistochemistry, which confirmed that 59 had ER-positive and 8 had ER-negative lesions.
Patient flow diagram.
Patient Characteristics (n = 344)
The prior management at the time of study inclusion was as follows: 99 patients (29%) were receiving adjuvant endocrine therapy after curative surgery, 51 (15%) were receiving palliative endocrine therapy, 16 (5%) were receiving neoadjuvant chemotherapy, 10 (3%) were receiving palliative chemotherapy, 1 (0.3%) was receiving adjuvant chemotherapy, 1 (0.3%) was receiving adjuvant radiotherapy, 81 (23%) was under regular follow-up after completion of treatment, and 85 (25%) had newly diagnosed breast cancer.
18F-FES PET/CT revealed that 172 (50%) patients exhibited exclusively 18F-FES–positive lesions, 81 (24%) had solely 18F-FES–negative lesions, and 91 (26%) had both 18F-FES–positive and 18F-FES–negative (mixed) lesions (Supplemental Fig. 1A). The most common clinical indication for 18F-FES PET/CT was to evaluate ER status when other imaging tests were equivocal (n = 105 [31%]), as shown in Supplemental Figure 1B. In detail, the most common equivocal lesions on standard workup were in bone (n = 42), followed by lung (n = 36) and lymph node (n = 30). CT was the most common standard workup showing equivocal results (n = 62), followed by 18F-FDG PET/CT (n = 29), bone scintigraphy (n = 28), and MRI (n = 5).
Clinical Impact of 18F-FES PET/CT
The results of the questionnaire for assessing the clinical impact of 18F-FES PET/CT were as follows: category 1 in 0 patients, category 2 in 85 (25%) patients, category 3 in 139 (40%) patients, category 4 in 10 (3%) patients, and category 5 in 110 (32%) patients. The management plans changed after 18F-FES PET/CT in 120 patients (35%; 95% CI, 30%–40%; category 4 or 5), with 18F-FES PET/CT considered to play a pivotal role in the beneficial change in most cases (110 [32%] with category 5). In addition, 18F-FES PET/CT was helpful in the clinical decision-making for 259 (75%; 95% CI, 70%–80%; category 3, 4, or 5) patients because the clinicians reported that it supported their decision in 139 patients (40%; category 3) even if it did not alter the actual treatment plan. The types of management before and after 18F-FES PET/CT are summarized in Supplemental Table 1. A Sankey diagram illustrating the comprehensive flow of management changes after 18F-FES PET/CT is available in Supplemental Figure 2.
Breakdown of Management Change Brought About by 18F-FES PET/CT
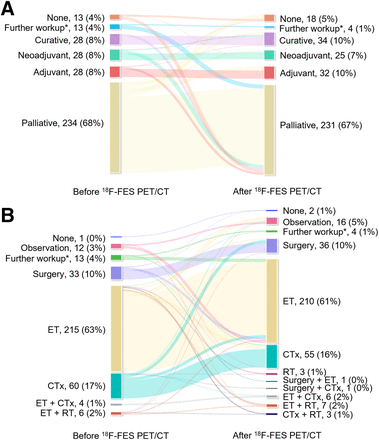
The proportion of intention-to-treat change was 22% (77/344; 95% CI, 18%–27%), accounting for 64% (77/120) of all 18F-FES PET/CT–induced changes (Fig. 2A). Specifically, 18F-FES PET/CT resulted in a shift from an intention-to-treat to palliative aim in 24 of 97 patients (25%) previously intended for nonpalliative (none, curative, neoadjuvant, or adjuvant) management. By contrast, 18F-FES PET/CT led to a change from a palliative to a nonpalliative treatment intent in 35 of 234 patients (15%) whose previous management intent was palliative. An interdisciplinary change accounted for 24% (82/344; 95% CI, 19%–29%), comprising 68% (82/120) of all changes. Although the changes in treatment modalities varied (Fig. 2B), the proportion of local treatment including surgery and radiotherapy, or systemic therapy combined with local treatment, showed an increase compared with previous policies (11% [39/344] to 15% [51/344]).
Sankey diagrams showing change in management before and after 18F-FES PET/CT with respect to intention-to-treat (A) and interdisciplinary (B) changes. CTx = chemotherapy; ET = endocrine therapy; RT = radiotherapy *Includes additional physical examination, laboratory tests, imaging studies, or biopsy.
The results of the questionnaire on the clinical impact of 18F-FES PET/CT according to the PET/CT findings and the clinical indications are shown in Supplemental Figures 3 and 4, respectively. The highest rate of change occurred in 18F-FES–negative cases (44% [36/81]; 95% CI, 33%–56%) followed by mixed 18F-FES–positive/negative cases (36% [33/91]; 95% CI, 26%–47%) and 18F-FES–positive cases (30% [51/172]; 95% CI, 23%–37%); however, no statistically significant difference was found (P = 0.067). A representative case in which negative 18F-FES uptake led to a change in management is shown in Figure 3. The frequency of management change was related to the clinical indications (P = 0.006); the highest rate of change was shown when evaluating the origins of metastasis in the case of double primary cancers (64% [9/14]; 95% CI, 35%–87%), followed by assessment of the ER status of lesions that were difficult to biopsy (45% [29/65]; 95% CI, 32%–58%). Representative cases in which 18F-FES PET changed the management plan for the aforementioned clinical indications are shown in Figures 4 and 5.
A 50-y-old woman diagnosed with ER-positive invasive ductal carcinoma in her left breast visited our hospital for surgical consultation. During initial workup, multiple sclerotic bone lesions were observed on chest and abdominopelvic CT scans (A, arrows). 18F-FES PET showed uptake in left breast tumor (B, arrow; SUVmax, 6.0) but no abnormal uptake in multiple bone lesions (B). Palliative endocrine therapy was initially considered, but curative surgery was performed because of findings on 18F-FES PET/CT. Follow-up CT scans 2 y after surgery showed no significant change in multiple bone lesions, suggesting that these were benign lesions such as osteopoikilosis, rather than multiple ER-negative metastases.
A 64-y-old woman was initially diagnosed with ER-positive invasive lobular carcinoma in her left breast. She underwent breast-conserving surgery followed by radiotherapy and was receiving adjuvant endocrine therapy. During routine follow-up, left lung nodule was incidentally detected on chest CT scan and subsequently biopsied, resulting in diagnosis of primary lung cancer. 18F-FDG PET/CT revealed hypermetabolic nodule in left lung (A, red arrows; SUVmax, 11.6) and another hypermetabolic lesion at left humerus (A, white arrows; SUVmax, 3.7). 18F-FES PET/CT showed no abnormal uptake by primary lung cancer in left lung (B, red arrows) and increased uptake in left humeral head (B, white arrows; SUVmax, 3.9), indicating ER-positive metastasis from breast cancer. Subsequent bone biopsy also confirmed metastasis from breast cancer. Although palliative chemotherapy for lung cancer was initially considered, management was shifted to surgical removal of lung cancer and palliative radiotherapy for humeral metastasis, along with palliative endocrine treatment with cyclin-dependent kinase 4/6 inhibitor.
An 80-y-old woman receiving adjuvant endocrine therapy after mastectomy for ER-positive invasive ductal carcinoma in her right breast developed left hemiparesis 2 wk previously. Brain MRI showed rim-enhancing cystic mass in right frontal lobe (A), suggesting brain metastasis. As biopsy was not feasible, 18F-FES PET/CT was performed, which did not show uptake (B). Instead of palliative surgery for metastatic lesion, curative resection for primary brain tumor was performed because of 18F-FES PET/CT findings; surgical specimen confirmed diagnosis of glioblastoma.
DISCUSSION
This study showed that 18F-FES PET/CT influenced the clinician’s management decisions for patients with breast cancer and provided an additional clinical impact over conventional workup modalities. Overall, 18F-FES PET/CT supported clinical decisions in three quarters of cases and resulted in a management change in one third of patients—proportions that are comparable to those of previous studies. Liu et al. (11) showed that the application of 18F-FES PET/CT changed the management in 5 of 19 (26%) patients with de novo ER-positive breast cancer. In another study, by van Kruchten et al. (10), 18F-FES PET/CT changed the treatment in 16 (48%) of 33 patients. In patients presenting with clinical dilemmas, Boers et al. (12) found that 51% (51/100) had a change in their initial management plan, and Sun et al. (18) reported that 48% (16/33) of patients had a change in treatment decision after 18F-FES PET/CT. Yang et al. (19) indicated that 18F-FES PET/CT resulted in a change in treatment decision in 7 of 32 (22%) patients with double primary malignancies. Furthermore, the frequency of management change was similar to that of 18F-FDG PET/CT from the National Oncologic PET Registry (20), which supports its coverage by Medicare. Our study included the largest (to our knowledge) prospective cohort of 18F-FES PET/CT to date used in real-world practice after approval for clinical use, assessed the significance of management changes by intention-to-treat and interdisciplinary changes, and provided a comprehensive analysis of its impact across various clinical situations.
Of the management changes prompted by 18F-FES PET/CT, intention-to-treat and interdisciplinary changes accounted for about two thirds. In detail, 18F-FES PET/CT resulted in a change in intention-to-treat management to a palliative aim in 25% of patients with a previously nonpalliative aim, because of the detection of incidental metastases on 18F-FES PET/CT that were not found on standard workup. Conversely, 18F-FES PET/CT shifted management to a potentially curative aim in 15% of patients who were initially intended for palliative treatment, by determining the ER status of suspected metastatic lesions (18). An interdisciplinary change involves more than just a change in the extent or site of surgery or radiotherapy, being a change in the modality of treatment, clinician, or department performing the treatment. Our results indicate that 18F-FES PET/CT can help avoid the adverse effects of unwarranted treatment and help select the optimal treatment by helping to reduce the potential under- or overestimation of tumor burden that might occur when evaluation is based solely on conventional workup modalities.
Regarding the results of 18F-FES PET/CT, we found that 18F-FES–negative or mixed 18F-FES–positive/negative results had a greater impact on clinical decision-making than 18F-FES–positive results. 18F-FES PET/CT can determine the ER status of lesions with high sensitivity and specificity in comparison with immunohistochemistry as the reference standard (9,16). 18F-FES–negative results can be interpreted as ER-negative metastasis or the absence of suspected lesion, and either of these may be contrary to the expectation based on standard workup and have a significant clinical impact. In addition, with the predictive role of 18F-FES PET/CT as a tool able to show function rather than just the presence of ER (7,21), there is a growing body of evidence showing that the presence of 18F-FES–negative lesions may indicate a poor response to endocrine therapy (22–24). Therefore, we believe that the impact of 18F-FES–negative results on clinicians’ decisions will increase. When we stratified the impact of 18F-FES PET/CT according to clinical indication, we found that 18F-FES PET/CT was most likely to result in a management change in patients with double primary cancers or in those for whom ER status was not determined because of difficult-to-biopsy lesions, highlighting the clinical utility of 18F-FES PET/CT for assessing ER status regardless of lesion location. In addition, 18F-FES PET/CT resulted in a change in management plan according to clinical indications ranging from 15% (staging of extraaxillary lymph nodes or distant metastases) to 64% (evaluating the origins of metastasis in the case of double primary cancers) of patients, as described in Supplemental Figure 4. These results support the appropriate-use criteria recently published by the Society of Nuclear Medicine and Molecular Imaging by showing the impact of 18F-FES PET/CT on clinical decisions according to the provided clinical scenarios (13).
Our study had several limitations. First, our analysis was retrospective, and retrospective evaluations of the impact of 18F-FES PET/CT based on reviews of medical charts may differ from prospective evaluations. However, the potential bias may be mitigated by the fact that all medical charts were recorded in a multidisciplinary clinic or by physicians specialized in breast cancer and the study population was from a prospective cohort. Second, although our institution has a multidisciplinary team specialized in recurrent or metastatic breast cancer, not all patients received multidisciplinary care, and not all the treatment decisions were made by a multidisciplinary team. Detailed changes in management, such as change in technique or extent of surgery or radiotherapy, are difficult to evaluate retrospectively. If all these changes were included, the clinical impact of 18F-FES PET/CT could have been even greater. Third, we relied solely on visual analysis without semiquantitative analysis for the assessment of 18F-FES positivity. SUV is protocol-dependent; therefore, we could not apply known SUV criteria for judging 18F-FES positivity (i.e., SUVmax ≥ 1.5 (9,12)). Nevertheless, semiquantitative analysis of 18F-FES PET/CT may contribute to the reproducible analysis of 18F-FES positivity and further affect clinical decisions. Fourth, biopsy of recurrent or metastatic lesions was performed on only 27% of patients. However, biopsy is often not feasible, and 18F-FES PET/CT is a good alternative in such cases. Therefore, the recruitment of patients might have been biased toward patients with difficult-to-biopsy lesions. Finally, whereas 18F-FES PET/CT was shown to lead to changes in management in a high percentage of patients, it is unknown whether these changes translated into better survival or quality-of-life outcomes. A recent pilot study suggested that a change in the management of breast cancer from endocrine therapy to chemotherapy, based on 18F-FES scan–negative results, may improve patient survival (25); however, further research is needed to clarify this issue.
CONCLUSION
18F-FES PET/CT resulted in modification of the actual management of recurrent or metastatic breast cancer and can serve as an impactful imaging modality for determining the optimal treatment strategy.
DISCLOSURE
This work was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (HR18C0016), and by a fund from the research program of the Korea Medical Institute. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What is the clinical impact of 18F-FES PET/CT on the actual management of patients with recurrent or metastatic breast cancer?
PERTINENT FINDINGS: Of a total of 344 patients, 120 (35%) experienced an actual change in management after 18F-FES PET/CT compared with the previous plan based on standard workup. In addition, 18F-FES PET/CT helped clinical decisions in a total of 259 (75%) patients, including 139 (40%) patients for whom 18F-FES PET/CT supported the existing management decision.
IMPLICATIONS FOR PATIENT CARE: 18F-FES PET/CT could influence the management of recurrent or metastatic breast cancer, serving as an impactful imaging modality for determining the optimal treatment strategy.
Footnotes
Published online Oct. 3, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication April 7, 2024.
- Accepted for publication August 28, 2024.