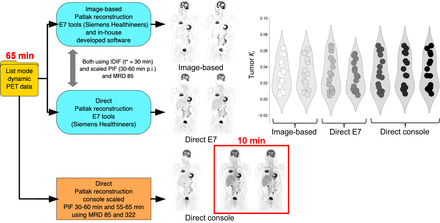
Visual Abstract
Abstract
Methods to shorten [18F]FDG Patlak PET imaging procedures ranging from 65–90 to 20–30 min after injection, using a population-averaged input function (PIF) scaled to patient-specific image-derived input function (IDIF) values, were recently evaluated. The aim of the present study was to explore the feasibility of ultrashort 10-min [18F]FDG Patlak imaging at 55–65 min after injection using a PIF combined with direct Patlak reconstructions to provide reliable quantitative accuracy of lung tumor uptake, compared with a full-duration 65-min acquisition using an IDIF. Methods: Patients underwent a 65-min dynamic PET acquisition on a long-axial-field-of-view (LAFOV) Biograph Vision Quadra PET/CT scanner. Subsequently, direct Patlak reconstructions and image-based (with reconstructed dynamic images) Patlak analyses were performed using both the IDIF (time to relative kinetic equilibrium between blood and tissue concentration (t*) = 30 min) and a scaled PIF at 30–60 min after injection. Next, direct Patlak reconstructions were performed on the system console using only the last 10 min of the acquisition, that is, from 55 to 65 min after injection, and a scaled PIF using maximum crystal ring difference settings of both 85 and 322. Tumor lesion and healthy-tissue uptake was quantified and compared between the differently obtained parametric images to assess quantitative accuracy. Results: Good agreement was obtained between direct- and image-based Patlak analyses using the IDIF (t* = 30 min) and scaled PIF at 30–60 min after injection, performed using the different approaches, with no more than 8.8% deviation in tumor influx rate value (Ki) (mean difference ranging from −0.0022 to 0.0018 mL/[min × g]). When direct Patlak reconstruction was performed on the system console, excellent agreement was found between the use of a scaled PIF at 30–60 min after injection versus 55–65 min after injection, with 2.4% deviation in tumor Ki (median difference, −0.0018 mL/[min × g]; range, −0.0047 to 0.0036 mL/[min × g]). For different maximum crystal ring difference settings using the scan time interval of 55–65 min after injection, only a 0.5% difference (median difference, 0.0000 mL/[min × g]; range, −0.0004 to 0.0013 mL/[min × g]) in tumor Ki was found. Conclusion: Ultrashort whole-body [18F]FDG Patlak imaging is feasible on an LAFOV Biograph Vision Quadra PET/CT system without loss of quantitative accuracy to assess lung tumor uptake compared with a full-duration 65-min acquisition. The ultrashort 10-min direct Patlak reconstruction with PIF allows for its implementation in clinical practice.
PET integrated with CT is established as a clinically routine diagnostic workup in oncology, including for initial diagnosis, staging, prognosis, radiation therapy planning, and evaluation of treatment response (1–3). [18F]FDG is the most used radiotracer and provides information on metabolically active processes in the body (4). The most common [18F]FDG PET acquisition mode in clinical routine is static imaging at 60 min after injection, which can be used to derive semiquantitative SUVs for tumor semiquantification and serves as a surrogate of tumor metabolic activity. Standardization methods regarding patient preparation, PET image acquisition, reconstruction settings, and image analysis, such as described in the European Association of Nuclear Medicine procedure guidelines for tumor imaging (5), aim to largely mitigate SUV variability for repeatable and reproducible results. Nonetheless, SUV is unable to capture changes in plasma kinetics—because of, for example, altered kidney function due to treatment—or to distinguish between specific and nonspecific uptake, possibly causing a dissociation between actual tumor metabolic activity and inaccurate SUV measurements (6–8). This dissociation may impair reliable visual and quantitative interpretation of, for example, baseline versus follow-up [18F]FDG PET images for evaluating response to treatment.
On the other hand, a dynamic PET acquisition measuring spatiotemporal activity concentration could include this information by providing voxelwise metabolic information after application of full kinetic or Patlak analyses (9–11).
Long-axial-field-of-view (LAFOV) PET systems capture the heart and all other organs of interest simultaneously and continuously using a single bed position. In addition, these systems, associated with high photon detection sensitivity, allow precise noninvasive measurements of the image-derived input function (IDIF) and tissue time–activity curves.
Previously, methods to shorten [18F]FDG Patlak PET imaging procedures, ranging from 65–90 min to 20–30 min after injection, using a population-averaged input function (PIF) scaled to patient-specific IDIF values, were evaluated (12–15). Previous investigations showed that the use of direct parametric reconstruction methods can lead to parametric images with significantly less noise (16,17). The aim of the present study was to investigate the feasibility of reducing scanning time even further and to assess the impact on quantification. We evaluated the feasibility of ultrashort 10-min [18F]FDG imaging (55–65 min after injection) for quantitatively accurate assessment of lung tumor uptake, using a PIF in combination with direct Patlak reconstructions and different maximum crystal ring differences (MRDs) on an LAFOV Biograph Vision Quadra PET/CT system (Siemens Healthineers). In addition, to validate the ultrashort 10-min direct Patlak reconstruction approach with application of a PIF, we compared these outcomes with a full-duration 65-min acquisition using various Patlak analysis methods, performed with both an IDIF and a PIF.
MATERIALS AND METHODS
Eleven clinically referred patients with suspected lung malignancy (lung cancer or lung metastases) were included in this study. The local Medical Ethics Review Committee of the University Medical Center Groningen waived the need for formal ethical review and the need to obtain informed consent for validation and optimization purposes using the Biograph Vision Quadra PET/CT (waiver METc2020/554). Following standardized European Association of Nuclear Medicine procedure guidelines for tumor imaging (5), we instructed patients to avoid strenuous exercise and fast for at least 6 h before receiving a weight-based injection of [18F]FDG (3 MBq/kg). A MEDRAD Intego PET infusion system (Bayer Pharmaceuticals) was used for automated [18F]FDG bolus injection. After a delay of 10 s to ensure that the PET list mode acquisition had started, an automated [18F]FDG bolus injection was performed using a MEDRAD Intego PET infusion system (Bayer Pharmaceuticals). Subsequently, a 65-min whole-body (from vertex to mid-thigh) dynamic list-mode PET scan was acquired on an LAFOV Biograph Vision Quadra PET/CT scanner. Dynamic PET data were binned over 31 frames using frame durations of 6 × 10, 3 × 20, 6 × 30, 5 × 60, and 11 × 300 s. In addition, patients underwent whole-body low-dose CT (with an x-ray tube current of 35 mAs, a tube voltage of 100 kV, and a spiral pitch factor of 1.1) for anatomic information and PET attenuation correction.
The reconstruction settings that were applied to the dynamic PET data were standards 2 of the European Association of Nuclear Medicine Research Ltd. (18), specifically a 3-dimensional ordered-subset expectation maximization algorithm with 4 iterations, 5 subsets, and a matrix size of 220 × 220 × 708 corresponding to a voxel size of 3.3 × 3.3 × 1.5 mm, with time-of-flight application, resolution modeling, and an isotropic 3-dimensional gaussian filter of 5 mm full width at half maximum. In addition, corrections for randoms, scatter, attenuation, and radioactive decay were used.
For the image-based Patlak analysis, a volume of interest (VOI) was placed in the ascending aorta to extract the IDIF (19). Subsequently, Patlak analyses were performed using both the IDIF (time to relative kinetic equilibrium between blood and tissue concentration (t*) = 30 min) and a scaled PIF at 30–60 min after injection using in-house–developed software. An averaged input function obtained from previously acquired representative data (acquisition and processing previously described (20)) was used as the PIF. The IDIF extracted from the dynamic images, as well as the PIF, were then included as input for the direct Patlak reconstructions at 30–60 min after injection using the E7 tools investigational reconstruction prototype software (Siemens Healthineers). The method to scale the PIF to patient-specific IDIF values, which has been applied for both the image-based and the E7 approaches incorporating a PIF, has previously been described (21). For the direct Patlak reconstructions on the system console (version VR20), the fully automated Patlak analysis feature Multiparametric PET AI (Siemens Healthineers) was applied. This fully automated approach incorporates ALPHA technology (22), which relies on landmarks such as the proximal descending aorta on CT and transfers these to the PET image to place a cylindric VOI (10-mm diameter, 20-mm height) in the lumen of the large vascular structure, automatically extracting the IDIF. Next, comprehensive direct parametric image estimation including scaling of an implemented PIF to the tail of the acquired patient-specific IDIF is conducted. The latter was performed for 30–60 min after injection, that is, 6 frames of 5 min, and for 55–65 min after injection, that is, 6 frames of 100 s. For the PET datasets including only the last 10 min of the acquisition, MRD settings of 85 and 322 were applied.
For comparison of quantitative accuracy, the net influx rate value (Ki) of tumor and healthy tissues was derived and compared between the differently obtained parametric images. For all images obtained using the different approaches, tumor and healthy-tissue segmentations were done using the ACCURATE tool (23). Tumors and gray matter were segmented using the semiautomated method (50% of peak isocontour). For healthy tissues, a spheric VOI of 3-cm diameter was placed in the liver, and 2-cm-diameter spheric VOIs were placed in the spleen and upper thigh muscle.
Variations in tumor Ki between the differently obtained parametric images, as well as variations in healthy-tissue Ki, were evaluated using scatterplots and violin plots. Linear regression analysis was conducted to assess the linear relationship between tumor Ki derived from the differently obtained parametric images. Furthermore, to assess the effect of Patlak reconstruction method on noise level, the SD in Ki derived from the liver VOI was compared among the different approaches. Bland–Altman plots were included to test the agreement in tumor Ki obtained using the different approaches. Statistical analyses were performed in SPSS Statistics, version 27.0 (IBM), and Excel (Microsoft).
RESULTS
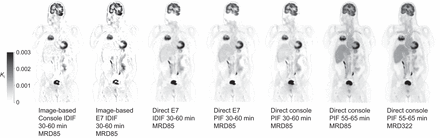
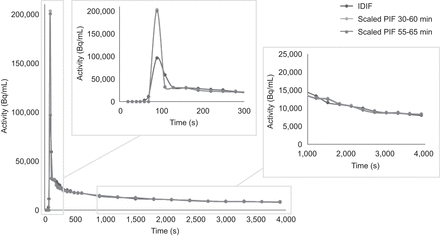
Eleven clinically referred oncologic patients (7 men, 4 women; age range, 64–80 y [mean ± SD, 71 ± 5 y]) with suspected lung malignancy were included and received a weight-based [18F]FDG injection (weight range, 69–134 kg [mean ± SD, 86 ± 18 kg]; activity range, 206–402 MBq [mean ± SD, 256 ± 54 MBq]). In total, 16 tumors (11 lung tumors and 5 pulmonary lymphoma lesions) were segmented, as well as healthy gray matter, liver, spleen, and muscle tissue. Patient demographics and specific injection parameters can be found in Table 1. Figure 1 provides examples of parametric Ki patient images illustrating image quality obtained with the various reconstruction and Patlak analysis approaches at different scan time intervals after injection, using both the IDIF and the scaled PIF and the different MRD settings. An example of a used IDIF and scaled PIF at 30–60 and 55–65 min after injection is illustrated in Figure 2.
Patient Demographics
Example coronal parametric Ki images of 65-y-old man with non–small cell lung cancer. Images were obtained with different approaches using IDIF or scaled PIF at different intervals after injection.
Example of used IDIF and scaled PIF at 30–60 and 55–65 min after injection.
Figure 3 shows violin plots of tumor Ki obtained with the different approaches; all methods result in a comparable Ki. Similarly, for healthy-tissue Ki comparison, violin plots are shown in Figure 4. Regarding healthy tissues, a clear difference in Ki obtained with the 55- to 65-min interval and scaled PIF approaches with respect to the other methods can be observed for the liver and spleen. Also for muscle tissue, Ki obtained with the scaled PIF at 55–65 min is slightly elevated. For gray matter, all reconstruction and analysis approaches result in a comparable Ki.
Violin plots of tumor Ki obtained with different reconstruction and analysis approaches using both IDIF and PIF at different intervals after injection.
Violin plots of healthy-tissue Ki (gray matter [A], liver [B], spleen [C], and muscle [D]) obtained with different reconstruction and analysis approaches using both IDIF and PIF at different intervals after injection. Vertical axis scale is adjusted for each individual organ to best illustrate differences in Ki.
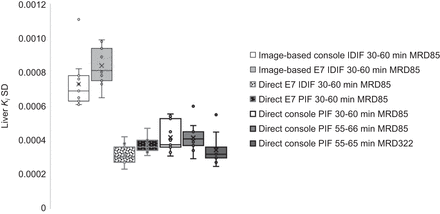
Figure 5 illustrates differences in liver Ki SD between the different approaches; a clear difference can be seen between the image-based (indirect) Patlak reconstruction methods and the direct Patlak reconstruction methods.
Box plots of liver Ki SD obtained with different reconstruction and analysis approaches using both IDIF and PIF at different intervals after injection.
A scatterplot comparing tumor Ki obtained using dynamic image reconstruction (image-based) and a subsequent offline Patlak analysis method with the IDIF (t* = 30 min) versus tumor Ki obtained using various other approaches is shown in Figure 6. Bias in tumor Ki was approximately 11% when using direct Patlak reconstruction and a scaled PIF on the system console for scaling intervals of both 30–60 and 55–65 min after injection. Furthermore, the agreement in tumor Ki among the different approaches is illustrated with Bland–Altman plots (Supplemental Fig. 1; supplemental materials are available at http://jnm.snmjournals.org). For the agreement between Ki obtained through image-based Patlak reconstruction using in-house–developed software versus image-based Patlak reconstruction using the E7 tools and an IDIF with a t* of 30 min after injection, the upper and lower limits of agreement (LOA) were −0.003 to 0.003 and the bias was −0.000. Regarding the agreement between Ki obtained through image-based Patlak reconstruction using in-house–developed software versus direct Patlak reconstruction on the E7 tools using an IDIF with a t* of 30 min after injection, the upper and lower LOA were −0.006 to 0.006 and the bias was −0.002. For assessing the agreement between Ki obtained through image-based Patlak reconstruction using an IDIF with a t* of 30 min and in-house–developed software versus direct Patlak reconstruction on the E7 tools using a PIF scaled at 30–60 min after injection, the Bland–Altman analysis showed upper and lower LOA of −0.005 to 0.005 and a bias of −0.002. With respect to agreement between Ki obtained through image-based Patlak reconstruction using in-house–developed software, an IDIF with a t* of 30 min, and in-house–developed software versus direct Patlak reconstruction on the console with a PIF scaled to the IDIF at 30–60 min after injection, the upper and lower LOA were −0.006 to 0.006 and the bias was −0.002. For assessing the agreement between Ki obtained through image-based Patlak reconstruction using an IDIF with a t* of 30 min and in-house–developed software versus direct Patlak reconstruction on the console with a PIF scaled to the IDIF at 55–65 min after injection and an MRD setting of 85 and 322, the upper and lower LOA were −0.008 to 0.008 and the bias was −0.004.
Scatterplots of tumor Ki derived from parametric images obtained with direct Patlak reconstruction on E7 tools using IDIF at 30–60 min after injection versus tumor Ki was obtained using various other approaches. Dashed lines represent linear regression fit with intercept set to (0,0), with corresponding linear regression equations and R2.
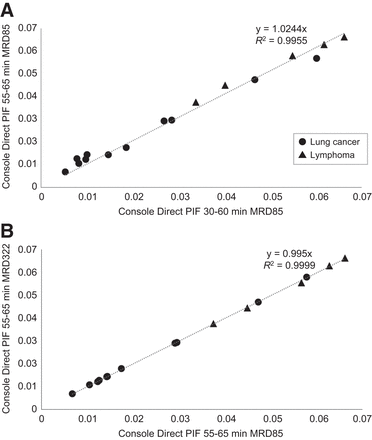
Figure 7 shows scatterplots comparing tumor Ki obtained using the direct Patlak reconstruction method on the console using a scaled PIF at 30–60 versus 55–65 min after injection, as well as comparing tumor Ki obtained using the direct Patlak reconstruction method on the console at 55–65 min after injection applying both MRD settings. When the scan duration was shortened from 30–60 to 55–65 min after injection, a highly correlated tumor Ki was found (R2 > 0.99), with 2.4% bias (median difference, −0.0018; range, −0.0047 to 0.0036). When MRD 85 versus MRD 322 was compared using the interval of 55–65 min after injection, tumor Ki was also highly correlated (R2 > 0.99), and the linear regression fit between the 2 approaches resulted in a difference of 0.5% (median difference, 0.0000; range, −0.0004 to 0.0013).
Scatterplot of tumor Ki derived from parametric images obtained on system console using PIF scaled to IDIF at 30–60 vs. 55–65 min after injection (A), as well as using PIF scaled to IDIF at 55–65 min after injection using MRD settings 85 vs. 322 (B). Dashed line represents linear regression fit with intercept set to (0,0).
DISCUSSION
Previously, possibilities have been described to reduce scan time for noninvasive whole-body [18F]FDG Patlak imaging using a PIF from 60–90 to 20–30 min (12–15,21). However, the introduction of LAFOV PET not only allowed for continuous whole-body dynamic imaging because of the larger field of view but also enabled a significant reduction in static whole-body PET scan duration to approximately 1 min because of the substantially increased sensitivity (24–27). Thus, whereas 20–30 min of dynamic imaging are a vast improvement over 60–90 min, a clinic running on high patient throughput would likely prefer an even shorter (static) scan. Therefore, in this work, we evaluated the feasibility of applying an ultrashort 10-min noninvasive whole-body [18F]FDG direct Patlak imaging protocol and assessed the obtained parametric Ki values on quantitative accuracy with respect to longer acquisition times, reconstruction methods, and Patlak analysis approaches, applying both the IDIF and the PIF for comparison.
In the present study, we incorporated various reconstruction and Patlak analysis methods at full and shortened scan durations, using both the IDIF and the PIF, and at different MRD settings. Regarding reconstruction and Patlak analysis methods, a clear difference between image-based and direct Patlak analysis can be observed (Figs. 1 and 4). Image-based Patlak analysis suffers from higher noise levels than direct Patlak reconstruction (28), as is confirmed by the larger liver Ki SDs. Between the direct Patlak reconstruction approaches, liver Ki SD remains relatively stable regardless of scan duration. This increase in noise between image-based and direct Patlak analysis may also have influenced the bias in tumor Ki as shown in Figures 2, 5, 6, and 7. Nonetheless, tumor Ki values are considered similar (mean bias, 8.2% and narrow LOA as illustrated in Fig. 6) among all different reconstruction, analysis, and scan duration approaches. For quantification of healthy tissues, there are some discrepancies in Ki among the various approaches, especially in the liver and spleen (Fig. 3). This mismatch in liver and spleen Ki among the different approaches could be related to violation of the Patlak assumption that [18F]FDG activity is irreversibly trapped in the cell (29). Especially at later time points after activity administration, dephosphorylation of [18F]FDG-6P may occur in the liver and spleen, leading to an underestimation in Ki. However, surprisingly, liver Ki obtained using the interval of 55–65 min after injection with a PIF on the system console are increased with respect to liver Ki obtained using the other approaches. A clear explanation for this phenomenon is currently lacking. In addition, the liver and spleen have high blood volume fractions, which are not considered by the Patlak approach and may affect Ki estimation (30). However, for the interval of 30–60 min, the direct Patlak reconstruction on the console provides consistent results with use of the E7 tools and image-based Patlak analysis, suggesting correct implementation of the Patlak model on the console. In addition, there is a slight increase in muscle Ki at 55–65 min after injection. Future studies using the generalized Patlak model, taking into account tracer dephosphorylation, should be conducted to explore possibilities of accurate healthy-tissue quantification using shorter scan time intervals at later time points after injection, as well as a comparison of results with, for example, compartment modeling or other parametric approaches.
In the current study, the automated direct Patlak reconstruction feature called Multiparametric PET AI is used with application of a PIF. This feature is Conformité Européenne–marked and available for Siemens PET/CT systems. The artificial intelligence component is merely the VOI placement for extraction of the IDIF. Even though the performance characteristics of PET/CT systems by other vendors would allow application of similar algorithms and make Multiparametric PET AI therefore translatable to other systems, other vendors would also have to go through the process of Conformité Européenne marking or Food and Drug Administration approval to implement these algorithms for clinical use. The specific feature as used in the current study implemented on the LAFOV Biograph Vision Quadra PET/CT cannot be copied and transferred to any other system, although the direct Patlak reconstruction itself could be implemented for any system. To our knowledge, other vendors have not yet included direct Patlak imaging together with a PIF in their optional clinical workflow imaging protocols. In addition, we do believe that ultrashort Patlak requires ultrasensitive PET/CT systems, as the overall short acquisition duration with short time frames requires images of good quality to capture the kinetics of [18F]FDG with sufficient precision. Yet, the ultrashort Patlak approach can be applied to any (future) ultra-high-sensitivity scanner, and most vendors are developing systems with axial fields of view of approximately 50 cm or more. We therefore expect that ultrashort Patlak may be feasible on any future clinical system.
Limitations of the current study are its small sample size and inclusion of only a single tumor type due to the long scan duration (65 min) of the initial dynamic dataset; including CT and patient positioning, the total scan duration was approximately 75 min. In addition, mismatches between PET and CT due to patient motion have not been evaluated or corrected for. These mismatches may influence quantitative accuracy but are not easy to correct for, particularly in direct reconstruction methods. Further optimization of the choice of nested iterations when short or low count frames are used may be considered before wider translation of direct Patlak imaging to clinical practice. Moreover, in future studies, it would be of interest to explore the evaluation of 10-min real-time whole-body Patlak imaging examinations in busy clinical routine on a larger scale in patients with various tumor types. In this way, the added clinical value of whole-body Patlak imaging could be assessed.
CONCLUSION
Using an LAFOV Biograph Vision Quadra PET/CT system, we found that ultrashort 10-min parametric whole-body [18F]FDG Patlak Ki imaging is feasible at the cost of minimal loss of quantitative accuracy and precision to assess lung tumor uptake, with respect to a full-duration 65-min acquisition. The implementation of a direct Patlak reconstruction algorithm with PIF, which requires only 10 min of scan time, allows for its implementation in clinical practice.
DISCLOSURE
Ronald Boellaard, Andor W.J.M. Glaudemans, Riemer H.J.A Slart, and Charalampos Tsoumpas receive research funds from Siemens Healthineers. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is ultrashort 10-min oncologic [18F]FDG whole-body Patlak imaging feasible?
PERTINENT FINDINGS: When the dynamic PET scan duration is reduced from 65 to 55–65 min after injection using a PIF, image quality is maintained without loss of quantitative accuracy to assess lung tumor uptake.
IMPLICATIONS FOR PATIENT CARE: Using an ultrashort noninvasive real-time 10-min [18F]FDG whole-body Patlak imaging protocol enables application in PET examinations during the busy clinical routine. This allows collection of large-scale datasets to assess the added value of parametric imaging in patient care.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication March 14, 2024.
- Accepted for publication August 28, 2024.