Visual Abstract
Abstract
C-X-C motif chemokine receptor 4 (CXCR4)–directed imaging has gained clinical interest in aiding clinical diagnostics in primary aldosteronism (PA). We retrospectively evaluated the feasibility of CXCR4-directed scintigraphy using the novel CXCR-4 ligand [99mTc]Tc-pentixatec in patients with PA. Methods: Six patients (mean age ± SD, 49 ± 15 y) underwent CXCR4-directed scintigraphy (including planar imaging and SPECT/CT) 30, 120, and 240 min after injection of 435 ± 50 MBq of [99mTc]Tc-pentixatec. Adrenal CXCR4 expression was analyzed by calculating lesion-to-contralateral ratios (LCRs). Imaging results were correlated to clinical information. Histopathology and clinical follow-up served as the standard of reference. Results: Three subjects showed lateralization of adrenal tracer accumulation, with a mean maximum lesion-to-contralateral ratio of 1.65 (range, 1.52–1.70), which correlated with morphologic findings on CT. One individual underwent adrenalectomy and presented with complete biochemical and clinical remission at follow-up. Histopathologic workup confirmed unilateral aldosterone-producing adenoma. Conclusion: [99mTc]Tc-pentixatec scintigraphy with SPECT in patients with PA is feasible and might offer a valuable alternative to CXCR4-directed imaging with [68Ga]Ga-pentixafor PET.
Primary aldosteronism (PA) is the most common cause of secondary arterial hypertension (1) and is characterized by increased aldosterone secretion resulting in arterial hypertension and reactively increased cardiovascular morbidity. In most cases, PA is caused by bilateral adrenal hyperplasia (idiopathic hyperaldosteronism [IHA]) or unilateral aldosterone-producing adenoma (APA). Differentiation is crucial, since PA can be cured by adrenalectomy in APA and treated by mineralocorticoid receptor antagonists (MRAs) in IHA.
Screening of selected patient groups with arterial hypertension is recommended by current endocrinologic guidelines. Measurement of plasma aldosterone concentration and renin levels and calculation of an aldosterone-to-renin ratio are used as screening tests for diagnosing PA. Imaging is performed for the detection of adrenal adenomas; however, morphologic imaging lacks further insight on the functional status of adenomas (2). Invasive catheterization in the form of adrenal vein sampling (AVS) remains the gold standard for differentiating subgroups of PA (3). However, the reliability and selective sampling of AVS highly depend on the experience of the performing physician (4). Noninvasive molecular imaging of C-X-C motif chemokine receptor 4 (CXCR4) expression has gained clinical interest (5). CXCR4 is a commonly expressed surface receptor on immune cells (5), and overexpression has been described for most hematologic and for several solid neoplasms (5,6). High CXCR4 expression has been reported for aldosterone-producing cells in APA (7).
Previous works have shown that CXCR4-directed PET/CT with [68Ga]Ga-pentixafor can facilitate therapeutic decision-making by differentiating APA from IHA (7,8). A prospective randomized, controlled trial investigating the accuracy of [68Ga]Ga-pentixafor PET/CT is currently ongoing (CASTUS, NL9625) (9).
The recent development of the novel 99mTc-labeled CXCR4 ligand [99mTc]Tc-pentixatec allows CXCR4-targeted conventional scintigraphy and SPECT/CT, providing a broader availability of CXCR4-directed imaging with improved cost-effectiveness (10,11). In the present study, we retrospectively evaluated the feasibility and diagnostic value of [99mTc]Tc-pentixatec scintigraphy/SPECT in patients with PA.
MATERIALS AND METHODS
This retrospective study was approved by the Ethics Committee of Ludwig Maximilians University of Munich (reference project number 23-0750) and adhered to the Declaration of Helsinki. All individuals were part of the German Conn’s registry and gave written informed consent to imaging procedures.
Between January 2022 and March 2023, 6 patients with PA were referred to our institution for CXCR4-directed scintigraphy with [99mTc]Tc-pentixatec. All subjects had initially undergone native CT imaging and AVS.
After adrenalectomy in 1 subject (patient 4), histopathologic work-up included immunohistochemical staining of CXCR4 (as described in the supplemental materials, available at http://jnm.snmjournals.org). In 4 patients, clinical follow-up was available 12 mo afterward. Two patients (patients 1 and 6) were lost to follow-up. Therapy response was assessed according to PA surgical outcome criteria (12) or stimulated renin levels (13). The supplemental materials provide further information.
[99mTc]Tc-Pentixatec Scintigraphy
Scans were performed using a dual-head γ-camera system (Discovery NM/CT 670 Pro; GE HealthCare). Patients underwent scintigraphy after intravenous injection of a mean of 435 ± 50 MBq (range, 377–511 MBq) of [99mTc]Tc-pentixatec. Planar whole-body scans were obtained 30, 120, and 240 min after tracer injection using a scan velocity of 30 cm/min (30-min scan) and 12 cm/min (120- and 240-min scans), respectively. A 20% energy window was centered over the 140-keV photopeak of 99mTc, as well as a lower scatter window ranging from 114 to 126 keV. After acquisition of planar scans, a SPECT/CT emission/transmission study was performed (180° acquisition per detector, 128 × 128 matrix, 3° steps, 8 s/frame for the 30- and 120-min SPECT/CT scans, and 16 s/frame for the 240-min scan). Images were reconstructed using an iterative ordered-subsets expectation maximization algorithm (10 subsets, 2 iterations), both with and without CT-based attenuation correction.
Transmission and emission images were fused on a dedicated nuclear medicine workstation (syngo.via; Siemens Healthcare).
Image Analysis
Images were visually assessed by 2 board-certified nuclear medicine physicians for asymmetry of tracer accumulation in the adrenal glands. For semiquantitative analysis, a volume of interest was placed over the adrenal glands in all SPECT/CT images (30, 120, and 240 min), and maximum counts, peak counts, and mean counts per VOI were extracted for each imaging time point. SUVmax, SUVpeak, and SUVmean were calculated offline using the patient’s weight and volume (density, 1 g/cm3), the injected activity, the time to scanning after tracer injection, and the calibration factor for 99mTc for the used SPECT/CT camera.
Adrenal lesion-to-contralateral ratios (LCRs) for SUVmax, SUVmean, and SUVpeak were calculated by dividing the higher SUV by the SUV of the contralateral side to derive imaging LCRs, in accordance with CXCR4-directed PET/CT imaging of PA (8,14). Lateralization was described for an LCR of 1.3 and above.
Statistical Analysis
Continuous variables are shown as mean ± SD or range. Statistical analyses were performed using SPSS Statistics, version 29.0.0.0 (IBM Corp.)
RESULTS
Patient characteristics and individual laboratory findings are displayed in Table 1. Six patients (4 male, 2 female; age, 49 ± 15 y) were included. Mean systolic blood pressure at the initial consultation was 151 ± 14 mm Hg. All patients were pretreated with noninfluencing antihypertensive medication including doxazosin and verapamil.
Distinct Laboratory Findings of Included Patients Before [99mTc]Tc-Pentixatec Scintigraphy
Individual findings from CT and AVS are displayed in Table 2.
Distinct Findings of CT, AVS, and [99mTc]Tc-Pentixatec Scintigraphy
[99mTc]Tc-Pentixatec Scintigraphy and Correlation to CT and AVS
CXCR4-directed scintigraphy was well tolerated by all patients, with no adverse effects recorded. Two of 6 patients showed consistent lateralization of adrenal tracer accumulation over all time points (Supplemental Table 1), with adenoma sizes of 16 and 19 mm. Patient 4 displayed inconsistent but clearly visible lateralization (maximum LCR, 1.52; adenoma size, 18 mm) (Fig. 1). Overall, a mean maximum LCR of 1.62 (range, 1.52–1.70) could be recorded in these individuals. Maximum LCRs were reached at 240 min after injection in all subjects. In the remaining 3 patients, no distinct lateralization of [99mTc]Tc-pentixatec uptake could be detected (Supplemental Table 1).
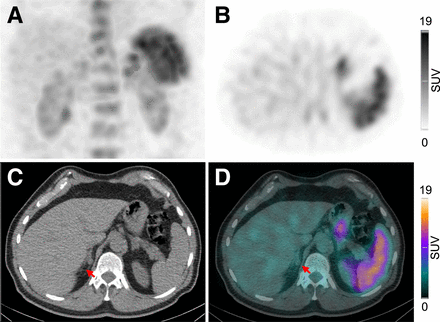
[99mTc]Tc-pentixatec scintigraphy and histopathologic findings of patient 4. Maximum-intensity projection (A), axial SPECT (B), CT (C), and fused SPECT/CT (D) images of abdomen depict intensive CXCR4 expression in left adrenal gland (arrows), corresponding to CT-graphic finding of 18-mm adenoma in left adrenal gland.
Focally enhanced tracer retention correlated with adrenal adenomas as detected by CT (mean size, 17.7 mm; range, 16–19 mm) in all 3 patients, whereas AVS did not detect any lateralization in 2 of 3 patients and produced inconsistent findings in patient 4. CT imaging revealed an 11-mm adenoma on the right adrenal gland in patient 6, which displayed no enhanced CXCR4 expression in CXCR4-directed imaging (Fig. 2).
[99mTc]Tc-pentixatec scintigraphy of patient 6. Maximum-intensity projection (A), axial SPECT (B), CT (C), and fused SPECT/CT (D) images of abdomen depict no lateralization of CXCR4 expression to either adrenal gland. Native CT imaging revealed nodular lesion (arrows) in right adrenal gland, which did not depict elevated CXCR4 expression, consistent with non–aldosterone-producing adenoma. Patient received MRA treatment after [99mTc]Tc-pentixatec scintigraphy.
Follow-up
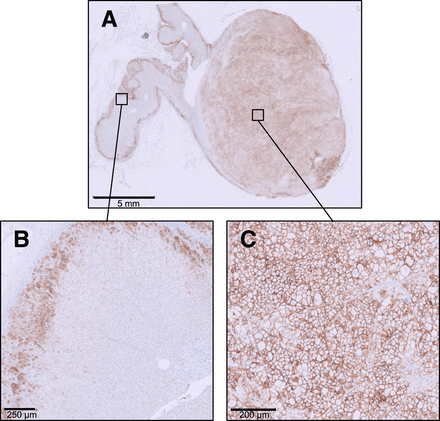
In consideration of clinical and diagnostic findings, adrenalectomy was recommended in patients 1 and 4. Patient 5 showed an equally high LCR, but AVS detected no lateralization despite selective sampling. Therefore, MRA treatment was recommended on an individual basis. Patient 1 refused surgery and requested MRA treatment. Thus, only patient 4 was referred for adrenalectomy of the left adrenal gland. Histopathologic work-up confirmed APA with a diameter of 17 mm and high CXCR4 expression (Fig. 3). At clinical follow-up 12 mo after surgery, all antihypertensive medication had been terminated in patient 4, indicating complete biochemical and clinical remission according to PA surgical outcome criteria (12). In the remaining patients, MRA therapy was started (Supplemental Table 2).
In patient 4, immunohistochemistry confirmed intense CXCR4 expression of APA (A and C) after adrenalectomy, and physiologic CXCR4 staining was detected in outer part of normal adrenal cortex (A and B).
DISCUSSION
Because treatment of APA and IHA greatly differs, easily accessible and noninvasive methods for the clinical differentiation of PA are urgently needed. CXCR4-directed PET/CT has emerged as a complementary noninvasive tool in PA diagnostics (7,8) and is being further investigated for aiding clinical decision-making.
In this retrospective analysis, we report on a first cohort of 6 consecutive patients with PA and inconclusive findings in prior diagnostic work-up who underwent CXCR4-directed scintigraphy with [99mTc]Tc-pentixatec. Three of 6 patients presented with a lateralization to either side, which was already visually noticeable at 30 min after tracer injection.
One patient was referred for adrenalectomy, which confirmed the diagnosis of CXCR-expressing APA. Two patients showed no adequate response to MRA treatment at follow-up, possibly because of an insufficient dose of MRA. Furthermore, an additional primary hypertension component is often present in patients requiring additional antihypertensive medication. Patient 5 had demonstrated an LCR consistent with APA but no lateralization in AVS despite selective sampling. If no adequate treatment response is seen after increasing the MRA dose, adrenalectomy should be reevaluated.
Because scintigraphy is more broadly available than PET/CT imaging, [99mTc]Tc-pentixatec scintigraphy could offer a cost-effective, low-threshold imaging modality for differentiating APA from IHA. This proof-of-concept study provides the first evidence—to our knowledge—on the feasibility and utility of this imaging modality in the context of PA. However, several limitations must be acknowledged. We included a small sample size of 6 patients, and histologic proof of diagnosis was available in only one patient. The optimal time point for imaging with [99mTc]Tc-pentixatec could not be determined. In our study, the highest LCRs were reached after 4 h, suggesting that later imaging time points may be beneficial for diagnosing APA, but further studies addressing this topic are needed. Although different LCR cutoffs (ranging between 1.6 and 2.36 (8,14)) with high sensitivity and specificity have been proposed in CXCR4-directed PET/CT imaging, a defined cutoff has not yet been determined for [99mTc]Tc-pentixatec scintigraphy. In this proof-of-concept study, we considered relevant lateralization to be present if LCR was 1.3 or greater. However, this value is arbitrary and currently lacks histologic confirmation. All LCR values for SUVmax, SUVpeak, and SUVmean, were comparable. We advocate the use of a calculated SUVmax for the calculation of LCR in analogy to SUVmax in PET/CT imaging (7,8,14).
Last, all suspected adenomas were more than 1 cm in size in the longest diameter, and APAs with greater diameters presented with higher LCRs. As many APAs are smaller than 1 cm, the sensitivity of SPECT/CT, with its limitations in spatial resolution, needs to be further evaluated in this subcohort of patients (15).
This proof-of-concept study imaging with [99mTc]Tc-pentixatec showed promising results in aiding clinical decision-making in PA. Further prospective studies on larger cohorts are needed to validate these findings and to define both optimal imaging protocols and quantitative cutoffs for lateralization.
CONCLUSION
Noninvasive visualization of CXCR4 in patients with PA using [99mTc]Tc-pentixatec is safe and feasible. This technique offers a valuable alternative to CXCR4-directed [68Ga]Ga-pentixafor PET/CT with broader availability for complementing classic diagnostic work-up in PA. Further research is warranted to corroborate its diagnostic performance, especially in comparison to CXCR4-directed PET/CT.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is [99mTc]Tc-pentixatec scintigraphy feasible in the diagnostic work-up of PA?
PERTINENT FINDINGS: In this retrospective evaluation, 3 of 6 patients undergoing [99mTc]Tc-pentixatec scintigraphy showed lateralization of adrenal CXCR4 expression. One patient was referred for adrenalectomy, and histopathology confirmed the diagnosis of unilateral APA.
IMPLICATIONS FOR PATIENT CARE: CXCR4 visualization in patients with PA using [99mTc]Tc-pentixatec is feasible. It might offer a valuable alternative to CXCR4-directed [68Ga]Ga-pentixafor PET/CT.
Footnotes
Published online Sep. 5, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication June 2, 2024.
- Accepted for publication August 13, 2024.