Visual Abstract
Abstract
Fibroblast activation protein (FAP) is abundantly expressed in the stroma of most human solid tumors. Clinical-stage radiolabeled FAP ligands are increasingly used as tools for the detection of various cancer lesions. To unleash the full therapeutic potential of FAP-targeting agents, ligands need to remain at the tumor site for several days after administration. We recently described the discovery of OncoFAP, a high-affinity small organic ligand of FAP with a rapid accumulation in tumors and low uptake in healthy tissues in cancer patients. Trimerization of OncoFAP provided a derivative (named TriOncoFAP, or OncoFAP-23) with improved FAP affinity. In this work, we evaluated the tissue biodistribution profile and the therapeutic performance of OncoFAP-23 in tumor-bearing mice. Methods: OncoFAP-23 was radiolabeled with the theranostic radionuclide 177Lu. Preclinical experiments were conducted on mice bearing SK-RC-52.hFAP (BALB/c nude mice) or CT-26.hFAP (BALB/c mice) tumors. 177Lu-OncoFAP and 177Lu-FAP-2286 were included in the biodistribution study as controls. Toxicologic evaluation was performed on Wistar rats and CD1 mice by injecting high doses of OncoFAP-23 or its cold-labeled counterpart, respectively. Results: 177Lu-OncoFAP-23 emerged for its best-in-class biodistribution profile, high and prolonged tumor uptake (i.e., ∼16 percentage injected dose/g at 96 h), and low accumulation in healthy organs, which correlates well with its potent single-agent anticancer activity at low levels of administered radioactivity. Combination treatment with the tumor-targeted interleukin 2 (L19-IL2, a clinical-stage immunocytokine) further expands the therapeutic window of 177Lu-OncoFAP-23 by potentiating its in vivo antitumor activity. Proteomics studies revealed a potent tumor-directed immune response on treatment with the combination. OncoFAP-23 and natLu-OncoFAP-23 exhibited a favorable toxicologic profile, without showing any side effects or signs of toxicity. Conclusion: OncoFAP-23 presents enhanced tumor uptake and tumor retention and low accumulation in healthy organs, findings that correspond to a strongly improved in vivo antitumor efficacy. The data presented in this work support the clinical development of 177Lu-OncoFAP-23 for the treatment of FAP-positive solid tumors.
- radiopharmaceuticals
- fibroblast activation protein
- OncoFAP
- radiopharmaceutical therapeutics
- targeted cancer therapy
- tumor microenvironment
Conventional cancer treatments are based on cytotoxic compounds that interfere with the biologic mechanisms involved in cell proliferation, thus preferentially acting on rapidly dividing cells, including cancer cells (1). Because of their untargeted nature, these drugs are not able to selectively accumulate at the tumor site and suffer from low efficacy against metastatic tumors and high systemic toxicity (2–4). Similarly to conventional chemotherapy, external-beam radiation therapy presents some major limitations. Although this therapeutic modality offers the possibility to focus a biocidal high-energy x-ray beam toward a cancer lesion, inducing DNA damage and cell death, it is hardly applicable to small lesions or diffuse and disseminated metastatic conditions (5–8). Additionally, along with common acute and transient side effects, radiotherapy may lead to the radiation-induced delayed development of new neoplastic lesions (9–11). The introduction of cancer immunotherapy, a modality that exploits the activation of the host immune system to be directed against tumor cells, resulted in a significant improvement in the efficacy and quality of life. However, these treatments are characterized by significant systemic toxicities and high variability of the response, especially in elderly patients with a weaker and less responsive immune system (12).
In the last decades, novel tumor-targeting strategies have been developed and implemented, aiming at broadening the therapeutic windows of anticancer payloads such as cytotoxic drugs (13,14) and radionuclides (15). Radiopharmaceutical therapeutics (RPTs) are emerging modalities composed of a tumor-homing moiety covalently connected to a radionuclide or a radiometal chelator (16–18). Thanks to the targeting vector, which binds to a tumor-associated antigen, RPTs can selectively deliver biocidal radiation (typically β−-particles or α-particles) to cancer cells anywhere in the body, resulting in high efficacy with reduced radioactive burden to healthy organs (19). RPTs are nowadays used in metastatic cancer patients who do not respond to earlier treatment lines (19). In the last few years, 2 RPTs based on 177Lu have gained market authorization for the treatment of metastatic castration-resistant prostate cancer (i.e., 177Lu-vipivotide tetraxetan [Pluvicto, a Novartis product]) (15,20) and neuroendocrine tumors (i.e., 177Lu-DOTATATE [Lutathera, a Novartis product]) (21,22), paving the way for this new class of drug. The therapeutic performance of RPTs depends on the quality of the molecular target, which should be overexpressed at the tumor site and absent from healthy structures, and on the ability of the ligand to bind the target of interest with high affinity, selectivity, and prolonged residence time (23). Several tumor-associated antigens have been exploited for various active delivery approaches. Still, most of these antigens are specific for certain tumor types, such as prostate cancer in the case of prostate-specific membrane antigen and neuroendocrine tumors in the case of somatostatin receptor 2 (20,21). Similarly to conventional radiotherapy, also RPTs might lead to the development of radiation-induced neoplastic lesions.
Fibroblast activation protein (FAP) is a serine protease overexpressed on the surface of cancer-associated fibroblasts. Those cells abundantly populate the stroma of most (i.e., >90%) solid human cancers. FAP expression is negligible in normal organs, making FAP an attractive tumor-associated antigen suitable for pan-tumoral applications (24–26). We previously reported the development of OncoFAP, a novel small organic ligand with subnanomolar affinity for FAP. 68Ga-labeled OncoFAP has been administered to more than 100 patients with different cancer indications under German 13.2b regulation (27,28). The diagnostic tracer is now being studied in a phase I clinical trial for the detection of solid tumors (28). Despite its excellent tumor-targeting performance as an imaging tool, these FAP ligands do not meet the stringent requirements on affinity and tumor-residence time needed for therapeutic applications, as the RPTs must stay in target lesions for several days to deliver enough DNA-damaging radiation (29).
The rational design of compact OncoFAP multimers, which accurately fit the wide and deep pocket of FAP, resulted in the identification of the trimeric OncoFAP-23 (half-maximal inhibitory concentration vs. FAP, 13 pM) (30). The new derivative was radiolabeled with 177Lu and tested on tumor-bearing mice for its biodistribution profile and anticancer efficacy. To further improve the therapeutic index and reduce the radioactive burden, we investigated combination treatments with the clinical-stage immunocytokine L19–interleukin 2 (L19-IL2) (31,32).
MATERIALS AND METHODS
Radiolabeling of OncoFAP Derivatives with 177Lu
The chemical structures of OncoFAP derivatives are reported in Figure 1. 177Lu radiolabeling of OncoFAP multimers was performed with different specific activities for the in vivo biodistribution and therapy studies. For the biodistribution study, precursors (100 nmol) were dissolved in 100 μL of Milli-Q water (MilliporeSigma) and diluted with 200 μL of sodium acetate (1 M in water, pH 4.5). 177Lu solution (20 MBq) was added, and the mixture was heated at 90°C for 10 min, followed by dilution with 1,600 μL of phosphate-buffered saline to achieve a final volume of 2 mL (20 doses of 100 μL each). Before the therapy studies, precursors (25 nmol) were dissolved in 25 μL of Milli-Q water, and then sodium acetate buffer (75 μL, 1 M in water) and 25, 75, or 150 MBq of 177Lu solution were added. The mixture was heated at 90°C for 10 min followed by dilution with 400 μL of phosphate-buffered saline to afford a final volume of 500 μL (5 doses of 100 μL each). Quality control of the radiosynthesis was performed using radio–high-performance liquid chromatography.
Chemical structures of OncoFAP-DOTAGA and OncoFAP-23-DOTAGA.
Animal Studies
All animal experiments were conducted in accordance with Swiss animal welfare laws and regulations under license ZH006/2021 granted by the Veterinäramt des Kantons Zürich.
Implantation of Subcutaneous Tumors
FAP-transfected tumor cell lines SK-RC-52.hFAP and CT-26.hFAP were previously generated by lentiviral transduction (33,34). FAP-positive tumor cells were cultured to 80% confluence in Dulbecco modified Eagle medium (Gibco) or RPMI-1640 (Gibco) with 10% fetal bovine serum (Gibco) and 1% antibiotic–antimycotic (Gibco) and detached with 0.05% trypsin-ethylenediaminetetraacetic acid. Cells were resuspended in Hanks balanced salt solution medium. Aliquots of 5 × 106 cells (100 μL of suspension) were injected subcutaneously in the flank of female athymic BALB/c AnNRj-Foxn1 mice (SK-RC-52.hFAP model; Janvier) or immunocompetent female BALB/c AnNRj mice (CT-26.hFAP model; Janvier).
Quantitative In Vivo Biodistribution Studies with Multivalent 177Lu-OncoFAP Conjugates
Tumors were allowed to grow to an average volume of 300–500 mm3. The mice were randomized (3 or 5 per group) and injected intravenously with the 177Lu-labeled FAP-targeting compounds (dose, 250 nmol/kg; 50 MBq/kg). The mice were euthanized by CO2 asphyxiation at different time points. Tumors, organs, and blood were harvested and weighed, and radioactivity was measured with a Packard Cobra γ-counter. Values are expressed as percentage injected dose per gram of tissue (%ID/g) ± SD.
In Vivo Therapeutic Efficacy of Multivalent 177Lu-OncoFAP Conjugates in Tumor-Bearing Mice
The in vivo anticancer activity of the different 177Lu-OncoFAP conjugates was assessed in athymic BALB/c AnNRj-Foxn1 mice bearing SK-RC-52.hFAP tumors and immunocompetent BALB/c AnNRj mice bearing CT-26.hFAP tumors. 177Lu-OncoFAP, 177Lu-BiOncoFAP, or 177Lu-OncoFAP-23 was intravenously administered at a dose of 250 nmol/kg, with 5, 15, or 30 MBq/mouse (a single administration). For combination therapies, a dose of RPT was followed by the administration of the clinical-stage immunocytokine L19-IL2 at a dose of 2.5 mg/kg. Therapy experiments started when the average volume of established solid tumors had reached 100–150 mm3. Animal body weight and tumor volume were measured daily. Animals were euthanized when one or more termination criteria indicated by the experimental license was reached. Prism 7 software (GraphPad Software) was used for data analysis.
Proteomic Analysis
Mice bearing SK-RC-52.hFAP or CT-26.hFAP subcutaneous tumors were treated with saline, 177Lu-OncoFAP-23 (250 nmol/kg, 250 MBq/kg), L19-IL2 (3 × 2.5 mg/kg), or the combination of 177Lu-OncoFAP-23 (250 nmol/kg, 250 MBq/kg) plus L19-IL2 (3 × 2.5 mg/kg). Animals were euthanized by CO2 asphyxiation at day 7 after the initiation of therapeutic treatments. Tumor samples were harvested and snap-frozen with liquid nitrogen. Tumor tissues were processed and analyzed as presented in the supplemental materials (available at http://jnm.snmjournals.org).
Immunofluorescence Staining
Detailed materials and methods for immunofluorescence staining are reported in the supplemental materials.
Safety and Toxicologic Evaluation in Rats and Mice
The safety and toxicity profile of OncoFAP-23 was evaluated in 2 separate studies performed in compliance with good-laboratory-practice and ICH M3(R2) guidelines. The first study (study A8) included the repeated administration of high doses of 175Lu-OncoFAP-23 (cold-labeled analog) in CD1 mice, whereas in the second study (study ST091) OncoFAP-23 (radiolabeling precursor) was administered as a single dose to Wistar rats. Detailed protocols and analyses are reported in the supplemental materials.
RESULTS
Quantitative In Vivo Biodistribution Studies with Multivalent 177Lu-OncoFAP Conjugates
177Lu-FAP-2286, a clinical-stage FAP ligand with a cyclic peptide structure (35), was included in the study as a benchmark. The monovalent OncoFAP analog is rapidly excreted (i.e., 2.5 %ID/g at 24 h and 1.9 %ID/g at 48 h in tumors), thus confirming its suitability as a radiodiagnostic—but not therapeutic—agent. 177Lu-OncoFAP-23 emerged for its superior biodistribution profile, presenting a high and prolonged uptake in target lesions at all tested time-points (e.g., 42 %ID/g at 24 h and 16 %ID/g at 96 h), with a favorable tumor-to-organ ratio (e.g., overall tumor-to-kidney ratio of 30, tumor-to-liver ratio of 62, and tumor-to-spleen ratio of 108) (Fig. 2; Supplemental Tables 1–8). As compared with 177Lu-OncoFAP-23, 177Lu-FAP-2286 (benchmark) presented a reduced tumor uptake (i.e., 10 %ID/g at 24 h and 5% at 96 h), with moderate trapping in the healthy kidney (tumor-to-kidney ratio of 6).
Results of quantitative in vivo biodistribution studies performed with 177Lu-OncoFAP (A), 177Lu-OncoFAP-23 (B), and 177Lu-FAP-2286 (C) (clinical-stage RPT benchmark) at different time points after intravenous administration to mice bearing SK-RC-52.hFAP tumors. All compounds were injected at equimolar doses and at same radioactive molar activity (dose = 250 nmol/kg; 50 MBq/kg; n = 3–5). hFAP = human fibroblast activation protein.
In Vivo Therapeutic Efficacy of 177Lu-OncoFAP Derivatives in Athymic BALB/c Nude Tumor-Bearing Mice
In a first therapy experiment (Fig. 3A), a single dose of 177Lu-OncoFAP, 177Lu-BiOncoFAP, or 177Lu-OncoFAP-23 was systemically administered to immunodeficient mice bearing SK-RC-52.hFAP tumors. All compounds were injected at a low activity corresponding to 250 MBq/kg (i.e., 5 MBq/mouse). Monotherapy with 177Lu-OncoFAP did not result in a significant therapeutic benefit. A minor tumor growth retardation was observed in mice treated with this compound over 20 d (average tumor volume at euthanasia, ∼455 mm3) as compared with the saline group (euthanized at day 16; average tumor volume, 453 mm3). Contrarily, 177Lu-BiOncoFAP and 177Lu-OncoFAP-23 induced a substantial anticancer effect, resulting, respectively, in average tumor volumes of 235 and 96 mm3 at the end of the therapy experiment (day 25).
In vivo therapeutic efficacy and percentage of body weight changes resulting from treatment with different 177Lu-OncoFAP compounds (alone or in combination with L19-IL2) in SK-RC-52.hFAP tumor–bearing mice. (A) 177Lu-OncoFAP (5 MBq/mouse, n = 4), 177Lu-BiOncoFAP (5 MBq/mouse, n = 4), and 177Lu-OncoFAP-23 (5 MBq/mouse, n = 4). (B) 177Lu-OncoFAP-23 (5, 15, or 30 MBq/mouse, n = 4), L19-IL2 (3 injections of 50 μg/mouse, n = 4), or their combination (n = 4). (C) 177Lu-OncoFAP-23 (5 MBq/mouse, n = 4), L19-IL2 (3 injections of 50 μg/mouse, n = 4), or their combination (n = 4) at different administration schedules (Combo S1–S4). Administration schedules are indicated with black arrows (177Lu-OncoFAP-23) and colored arrows (L19-IL2). C.R. = complete remission.
We further explored the therapeutic potential of 177Lu-OncoFAP-23, both in a monotherapy regimen at different activities (5, 15, and 30 MBq/mouse) and in combination with a clinical-stage tumor-targeted IL2 (L19-IL2). The latter already presented a strong synergy with 177Lu-BiOncoFAP (36). 177Lu-OncoFAP-23 induced a dose-dependent anticancer activity, with 1 of 4 complete remissions at the 15-MBq dose level, and 2 of 4 complete remissions in the 30-MBq group. The combination of a low radioactive dose of 177Lu-OncoFAP-23 (5 MBq/mouse) with L19-IL2 (3 × 50 μg/mouse) induced complete and durable cancer remissions in all treated animals (Fig. 3B).
In view of the clinical translation, we performed a schedule optimization by changing the timing (every 2 or 5 d) or number (1 or 3) of L19-IL2 doses (Fig. 3C). The therapeutic efficacy of the combination was independent of the spacing between the 3 L19-IL2 administrations, leading in both cases to complete cures in all treated animals. However, multiple administrations of L19-IL2 resulted in stronger antitumor activity, whereas a single injection of the combination product led to only 1 of 4 complete remissions in both cases. Two-way ANOVA statistical analyses for the therapy experiments displayed in Figures 3A–3C are reported in Supplemental Tables 9–11.
In Vivo Therapeutic Efficacy in Immunocompetent BALB/c Tumor-Bearing Mice
To further validate the therapeutic potential of the combination of 177Lu-OncoFAP-23 plus L19-IL2, we repeated the therapy studies in fully immunocompetent mice, using CT-26.hFAP murine colorectal carcinoma. This cell line–derived xenograft model was engineered to express low levels of FAP (33). Ex vivo proteomic analysis on tumor samples confirmed an approximately 5-fold lower abundance of FAP than in the SK-RC-52.hFAP xenograft (Fig. 4). The same trend was observed by a quantitative radioactive biodistribution analysis with 177Lu-OncoFAP-23, for which at 24 h the radioligand showed only about 10 %ID/g in CT-26.hFAP tumors, as compared with approximately 42 %ID/g in SK-RC-52.hFAP tumors (Fig. 4). As a result, single-agent treatment with low injected activities of 177Lu-OncoFAP-23 (250 nmol/kg, 250 MBq/kg) resulted in moderate anticancer efficacy (average tumor volume, 445 mm3 at day 23). Combination with L19-IL2 significantly improved the therapeutic index (average tumor volume, 28 mm3 at day 23), leading to strong anticancer efficacy and 3 of 4 complete remissions. Two-way ANOVA statistical analysis for the therapy experiments displayed in Figure 4C is reported in Supplemental Table 12.
(A) Results of quantitative in vivo biodistribution studies performed with 177Lu-OncoFAP-23 at 24 h after administration in 2 different tumor models (SK-RC-52.hFAP in athymic BALB/c nude mice or CT-26.hFAP in immunocompetent BALB/c mice). (B) Ex vivo relative FAP abundance in SK-RC-52.hFAP and CT-26.hFAP tumor samples, as assessed by proteomic analysis. (C) In vivo therapeutic efficacy and percentage of body weight changes after systemic administration of 177Lu-OncoFAP-23 (5 MBq/mouse, n = 4), L19-IL2 (3 injections of 50 μg/mouse, n = 4), or their combination (n = 4) in immunocompetent BALB/c mice bearing subcutaneous CT-26.hFAP tumors. Administration schedules are indicated with black arrows (177Lu-OncoFAP-23) and blue arrows (L19-IL2). C.R. = complete remission.
Proteomic Analysis
Proteomic analysis on tumor samples was conducted in parallel on both immunodeficient (BALB/c nu/nu–bearing subcutis SK-RC-52.hFAP xenografts) and immunocompetent (BALB/c-bearing subcutis CT-26.hFAP tumors) mouse models. The proteomic results depicted in the supplemental materials describe a pattern of cancer cell suppression mediated by proinflammatory tumor-infiltrating immune cells contributed by the host.
Immunofluorescence Staining
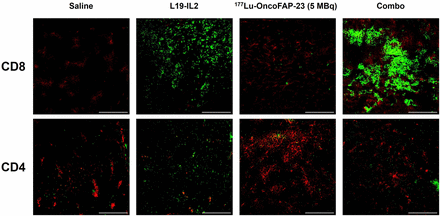
Ex vivo immunofluorescence staining of CT-26.hFAP tumor samples revealed a marked increase in CD8+ cells in tumors of mice treated with the combination of 177Lu-OncoFAP-23 plus L19-IL2. Single-agent treatment with L19-IL2 determined only a minor increase in CD8+ cells. Contrarily, CD4+ cells were not significantly more abundant in the treatment groups (Fig. 5).
Ex vivo immunofluorescence detection of CD4 and CD8 on CT-26.hFAP tumor sections after administration of 177Lu-OncoFAP-23 (single injection, 5 MBq/mouse), L19-IL2 (3 injections, 2.5 mg/kg), or their combination, following schedule indicated in Figure 4C. Representative immunofluorescence analysis of tumor samples is depicted at ×20 magnification. Green = CD8 or CD4 staining; red = CD31 staining (vasculature); scale bars = 100 μm.
Toxicology Studies on Mice and Rats
The toxicologic profile of OncoFAP-23-DOTAGA and 175Lu-OncoFAP-23 (cold-labeled analog of 177Lu-OncoFAP-23) was evaluated in rats and mice. Special focus was placed on the pharmacodynamic effects on organs other than the target site (tumor). Repeated injections of 175Lu-OncoFAP-23 at doses of 2.5 mg/kg (i.e., ∼1,000 nmol/kg) in CD1 mice (study A8) and a single injection of OncoFAP-23-DOTAGA in Wistar rats (study ST091), at the high dose of 20 mg/kg (i.e., ∼9,000 nmol/kg), resulted in no pathologic clinical signs of toxicity related to the treatment. In both studies, no relevant differences in body weight or food intake were observed between the groups. No clinically significant changes in blood biochemistry or hematology were observed. All animals were in good nutritional status, and no gross lesions were observed during necropsy. No significant differences in organ weight (liver and kidneys) were observed among the experimental groups. No histopathologic changes clearly attributable to the administration of OncoFAP-23-DOTAGA or 175Lu-OncoFAP-23 were observed (Table 1).
Safety and Toxicity Studies Compliant with Good-Laboratory-Practice and ICH M3(R2) Guidelines
DISCUSSION
Despite their excellent performance as radiotracers, diagnostic FAP ligands require further optimization to address therapeutic applications, as they clear from target lesions in a matter of a few hours after systemic administration (37,38). To allow time for their radioactive payload to kill cancer cells, the uptake of RPTs at the tumor site needs not only to be selective over healthy organs but to persist for several days. Various strategies to generate FAP ligands with a long tumor residence time have recently been pursued. Multivalent compounds such as DOTA.(SA.FAPI)2 (39) (dimer), ND-bisFAPI (40) (dimer), DOTA-2P(FAPI)2 (41) (dimer), and DOTA-4P(FAPI)4 (42) (tetramer) have demonstrated increased and prolonged accumulation at the site of disease as compared with their monovalent counterpart (i.e., FAPI-46). Similarly, previously described OncoFAP homodimers (i.e., BiOncoFAP) present a promising in vivo biodistribution profile with excellent tumor-to-organ ratios and potent anticancer therapeutic activity (29,36). We recently synthesized and tested various OncoFAP multimers (n = 2, 3, 4, 6, 8) for their in vitro inhibitory activity against FAP. Among the new multimers, the trivalent derivative (TriOncoFAP, also named OncoFAP-23) emerged for its superior biodistribution profile in a quantitative radioactive biodistribution analysis of the 177Lu conjugates in tumor-bearing mice (30). Thanks to its compact design, 177Lu-OncoFAP-23 presents low intrinsic trapping in excretory structures such as the liver and kidney, where bulkier multimers are prone to accumulate (30,43–45).
177Lu-OncoFAP-23 exhibits potent in vivo anticancer activity already at low radioactivity corresponding to a human equivalent dose of about 1.4 GBq/injection (5-fold lower than the recommended activity of marketed RPTs) (20,21). Moreover, 177Lu-OncoFAP-23 presents a much cleaner preclinical biodistribution profile than does 177Lu-vipivotide tetraxetan or 177Lu-DOTATATE (46–48), thus opening an opportunity to increase the administered activity over the canonic 7.4 GBq/injection (i.e., the clinical activity approved for 177Lu-DOTATATE and 177Lu-vipivotide tetraxetan) without incurring dose-limiting toxicities.
In this article, we have demonstrated that the therapeutic potential of 177Lu-OncoFAP-23 can be further boosted by combination with the clinical-stage immunocytokine L19-IL2 targeting the extracellular domain B of fibronectin in the tumor extracellular matrix and neovasculature. L19-IL2 has already shown potent synergism with various therapeutic modalities (36,49–55) in preclinical and clinical settings. After initial tumor damage induced by 177Lu-OncoFAP-23, L19-IL2 triggers a potent immune response directed toward the tumor lesions. Our proteomics (Supplemental Fig. 1) and immunofluorescence (Fig. 5) findings show strong activation of tumor-infiltrating natural killer cells (in athymic immunodeficient mice) and CD8 cells (in fully immunocompetent mice). Treatment with 177Lu-OncoFAP-23 in combination with L19-IL2 produces a strong synergistic effect that allows deescalation of the therapeutic dose of 177Lu-OncoFAP-23, thus further reducing the radioactive burden. Notably, this combination is highly effective also in a mouse model with low expression of FAP (Fig. 4), which might recapitulate the situation of certain cancer patient categories.
177Lu-FAP-2286, a novel FAP-targeted RPT based on a cyclic peptidic structure and now in a phase I/II clinical trial (LuMIERE trial), aims at evaluating the safety profile at activity levels ranging from 3.70 to 9.25 GBq/injection (35,56,57). Preliminary data from the study demonstrated a manageable safety profile and promising preliminary antitumor activity (58). In a separate study, a single injection of about 7.0 GBq of 177Lu-FAP-2286 resulted in potent anticancer activity in a patient with lung squamous cell carcinoma, with a significant radiographic response as monitored by 68Ga-FAP-2286 (59). When compared with 177Lu-FAP-2286, 177Lu-OncoFAP-23 exhibited an approximately 2.7-fold higher overall uptake in tumors, with an approximately 1.8-fold lower kidney uptake (i.e., a ∼5-fold better tumor-to-kidney ratio). This implies that this novel compound can deliver increased doses of radiation with decreased exposure to healthy organs, as compared with the most advanced FAP-targeted RPT in clinical development. On the basis of the preclinical evidence presented in this article, 177Lu-OncoFAP-23 therapy promises to outperform FAP-targeted radiopharmaceutical therapeutics based on different targeting moieties (e.g., monovalent ligands and cyclic peptides).
The data presented in this article strongly support the clinical development of 177Lu-OncoFAP-23, a trimeric FAP ligand with optimized tumor-targeting performance. Thanks to the availability of robust GMP radiolabeling procedures (60), a large batch of good-manufacturing-practice–grade OncoFAP-23-DOTAGA precursor, and safety data in rodents at high doses, our group is now launching a phase I clinical trial. The goal of the study is to define the 177Lu-OncoFAP-23 maximum tolerated dose, evaluate its safety profile, and collect preliminary signs of efficacy. The compound will be given as a single agent or in combination with L19-IL2 to patients with multiple types of FAP-positive tumors.
CONCLUSION
OncoFAP-23 presents enhanced tumor uptake and tumor retention and low accumulation in healthy organs, findings that correspond to a strongly improved in vivo antitumor efficacy. The data presented in this work support the clinical development of 177Lu-OncoFAP-23 for the treatment of FAP-positive solid tumors.
DISCLOSURE
Dario Neri is a cofounder and shareholder of Philogen, a Swiss-Italian Biotech company that operates in the field of ligand-based pharmacodelivery. Andrea Galbiati, Matilde Bocci, Domenico Ravazza, Jacqueline Mock, Ettore Gilardoni, and Samuele Cazzamalli are employees of Philochem AG, the daughter company of Philogen that owns and has patented OncoFAP derivatives. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What is the therapeutic performance of 177Lu-OncoFAP-23, a 177Lu-homotrimeric FAP ligand conjugate with ultrahigh affinity for the target?
PERTINENT FINDINGS: 177Lu-OncoFAP-23 displays a favorable biodistribution profile and potent in vivo anticancer activity in preclinical murine models. The antitumor properties of 177Lu-OncoFAP-23 are potently boosted by combination with L19-IL2, a clinical-stage tumor-targeted IL2.
IMPLICATIONS FOR PATIENT CARE: The favorable preclinical performance of 177Lu-OncoFAP-23 supports the clinical development of the radiopharmaceutical for the treatment of FAP-positive cancer lesions.
ACKNOWLEDGMENT
We thank Aureliano Zana for his help with setting up the radioactive experiments. We also wish to thank Farefarma Srl for their work on the toxicology evaluation of OncoFAP-23.
Footnotes
Published online Sep. 12, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication June 7, 2024.
- Accepted for publication August 5, 2024.