Visual Abstract
Abstract
Our purpose was to prospectively assess the distribution of NETPET scores in well-differentiated (WD) grade 2 and 3 gastroenteropancreatic (GEP) neuroendocrine tumors (NETs) and to determine the impact of the NETPET score on clinical management. Methods: This single-arm, institutional ethics review board–approved prospective study included 40 patients with histologically proven WD GEP NETs. 68Ga-DOTATATE PET and 18F-FDG PET were performed within 21 d of each other. NETPET scores were evaluated qualitatively by 2 reviewers, with up to 10 marker lesions selected for each patient. The quantitative parameters that were evaluated included marker lesion SUVmax for each tracer; 18F-FDG/68Ga-DOTATATE SUVmax ratios; functional tumor volume (FTV) and metabolic tumor volume (MTV) on 68Ga-DOTATATE and 18F-FDG PET, respectively; and FTV/MTV ratios. The treatment plan before and after 18F-FDG PET was recorded. Results: There were 22 men and 18 women (mean age, 60.8 y) with grade 2 (n = 24) or grade 3 (n = 16) tumors and a mean Ki-67 index of 16.1%. NETPET scores of P0, P1, P2A, P2B, P3B, P4B, and P5 were documented in 2 (5%), 5 (12.5%), 5 (12.5%) 20 (50%), 2 (5%), 4 (10%), and 2 (5%) patients, respectively. No association was found between the SUVmax of target lesions on 68Ga-DOTATATE and the SUVmax of target lesions on 18F-FDG PET (P = 0.505). 18F-FDG/68Ga-DOTATATE SUVmax ratios were significantly lower for patients with low (P1–P2) primary NETPET scores than for those with high (P3–P5) primary NETPET scores (mean ± SD, 0.20 ± 0.13 and 1.68 ± 1.44, respectively; P < 0.001). MTV on 18F-FDG PET was significantly lower for low primary NETPET scores than for high ones (mean ± SD, 464 ± 601 cm3 and 66 ± 114 cm3, respectively; P = 0.005). A change in the type of management was observed in 42.5% of patients after 18F-FDG PET, with the most common being a change from systemic therapy to peptide receptor radionuclide therapy and from debulking surgery to systemic therapy. Conclusion: There was a heterogeneous distribution of NETPET scores in patients with WD grade 2 and 3 GEP NETs, with more than 1 in 5 patients having a high NETPET score and a frequent change in management after 18F-FDG PET. Quantitative parameters including 18F-FDG/68Ga-DOTATATE SUVmax ratios in target lesions and FTV/MTV ratios can discriminate between patients with high and low NETPET scores.
Neuroendocrine tumors (NETs) are a heterogeneous group of tumors originating from enterochromaffin cells (1). The most common are gastroenteropancreatic (GEP) NETs, which are classified (World Health Organization, 2022) as well-differentiated (WD; 80%–90%) or poorly differentiated (10%–20%) neuroendocrine carcinomas. Grading of WD NETs (grades 1–3) is based on the mitotic index and the Ki-67 index (2). The site and extent of disease, as well as tumor grade, proliferative activity, and somatostatin receptor (SSTR) expression, impact the management of NETs. Systemic treatment is the cornerstone of treatment plans for patients with locally advanced or metastatic tumors. However, unlike in other solid tumors, options such as debulking surgery, embolization, and peptide receptor radionuclide therapy (PRRT) can be offered for progressive GEP NET patients (3–5).
The current staging strategy for NETs includes conventional cross-sectional imaging (CT with or without MRI) and SSTR PET imaging, most often with 68Ga-labeled somatostatin analogs such as DOTA-conjugated peptide (Tyr3)‐octreotate (68Ga-DOTATATE). 68Ga-DOTATATE uptake has been shown to be related to NET grade and is higher in WD NETs than in poorly differentiated neuroendocrine carcinomas. In addition, high avidity on 68Ga-DOTATATE PET as measured by SUVmax has been shown to correlate with a better response to PRRT and improved overall survival (6–8). Tumor metabolic activity can be assessed and quantified using 18F-FDG PET, with 18F-FDG avidity ranging from 40% in grade 1 tumors to 93% in grade 3. Generally, higher tumor metabolic activity (SUVmax > 10) is associated with poorer response to PRRT and poorer overall survival (9–13).
Patients with grade 2 and 3 WD GEP NETs show variable outcomes (14), making the selection of an optimal treatment strategy challenging. To date, the clinical impact of 68Ga-DOTATATE and 18F-FDG PET findings on the therapeutic strategy for NETs has been only retrospectively described (15–21). A dual-tracer 68Ga-DOTATATE/18F-FDG grading system developed from retrospective data was proposed by Chan et al. as a promising comprehensive imaging biomarker to better characterize WD metastatic NETs (22). This NETPET scoring algorithm considers tumor uptake on both 68Ga-DOTATATE and 18F-FDG PET. The main aims of the current study were to prospectively assess the distribution of NETPET scores (P1–P5) in grade 2 and 3 GEP NETs and to determine the impact of the NETPET score on the clinical management of these patients. Secondary aims included correlation of SUVmax on 68Ga-DOTATATE PET and 18F-FDG PET and correlation of quantitative measures on 68Ga-DOTATATE and 18F-FDG PET to NETPET scores.
MATERIALS AND METHODS
This institutional ethics review board–approved prospective, single-arm pilot study included 40 patients with histologically proven WD grade 2 or 3 GEP NETs or metastatic NETs of unknown primary clinically thought to be from the gastrointestinal tract, referred for baseline staging or restaging (NCT04804371). Written informed consent was obtained from all participants. The inclusion criteria were an age of at least 18 y, a tumor proliferation index (Ki-67 index) of at least 3%, and treatment-naïve or after any number of prior systemic therapy lines for metastatic disease or locally advanced inoperable tumor. The exclusion criteria were NETs not from a GEP primary site, WD grade 1 NETs or neuroendocrine carcinoma, and mixed neuroendocrine and nonneuroendocrine cancer. Patient screening is described in Figure 1.
Patient selection. *Patient was found to be ineligible for PRRT and was withdrawn from study by treating oncologist.
All eligible study participants underwent 18F-FDG PET within 21 d of 68Ga-DOTATATE PET. Demographic data (age, sex), tumor differentiation and grade, Ki-67 index, primary tumor site, and indication for exam (initial staging or restaging) were tabulated. Treatment intent (curative, palliative, observational) and specific management plan were recorded before 18F-FDG PET along with all available clinical and imaging information, including 68Ga-DOTATATE PET, and then again after 18F-FDG PET. The specific management plan included surgery (curative, debulking), liver-directed therapy, systemic therapy (e.g., somatostatin analogs, targeted agents, and chemotherapy), or PRRT.
PET Imaging
PET/CT was performed 61.9 ± 9.6 min (mean ± SD) after injection of 148.5 ± 31.6 MBq of 68Ga-DOTATATE and 61.9 ± 10.8 min after injection of 372.9 ± 61.2 MBq of 18F-FDG. During the uptake time, a water-soluble oral contrast agent was given for bowel opacification on CT. Patients were positioned supine with their arms outside the region of interest. PET/CT was performed on a Biograph mCT 40 scanner (Siemens Healthcare). Low-dose CT without an intravenous contrast agent was used for attenuation correction as per standard departmental protocols. Overall, 5–9 bed positions were obtained as per patient height (2–5 min/bed position). Further details on the imaging procedures and image interpretation criteria can be found in the European Association of Nuclear Medicine guidelines (23).
Data Interpretation and Analysis
The PET data were interpreted on a dedicated workstation (Thinking Systems PACSCloud; Thinking Systems Corp.) independently by 2 of 6 readers with 3–22 y of experience interpreting PET scans (median, 12 y). Sites of metastatic disease, NETPET score, and SUVmax on 68Ga-DOTATATE and 18F-FDG PET were recorded for up to 10 target lesions per patient, with no more than 3 target lesions for each organ. Readers determined the NETPET score after assessing both 68Ga-DOTATATE and 18F-FDG PET scans, following the methodology described by Chan et al. (22). In brief, images were assessed visually after the studies were scaled to an SUVmax scale from 0 to 15 for 68Ga-DOTATATE and from 0 to 7 for 18F-FDG. A primary NETPET score is derived on the basis of the most 18F-FDG–avid lesion relative to its SSTR uptake, as this is likely to represent the most aggressive phenotype of the disease present in the subject, excluding other non-NET causes of the increase in 18F-FDG uptake. The degree of 18F-FDG uptake compared with 68Ga-DOTATATE uptake in the target lesion defines the primary NETPET category on a 0–5 categoric scale, with P1 indicating purely SSTR-avid/18F-FDG–negative disease and P5 indicating significantly 18F-FDG–avid/SSTR-negative disease. P0 indicates normal scan findings on both SSTR PET and 18F-FDG PET. Then, the burden of disease is further characterized on the basis of the number of lesions exhibiting the traits in a secondary category (Fig. 2). For this study, a primary NETPET score (patient level), as well as a lesion NETPET score for all target lesions, was obtained. After independent review, disagreements on NETPET scores were assessed in a consensus meeting, and if no consensus could be reached a third reviewer would arbitrate.
NETPET classification scheme. Primary classification is in top row; secondary classification by lesion number is in bottom row. FDG = 18F-FDG.
Quantitative Data
The association between the SUVmax for both tracers for all target lesions was assessed. In addition to SUVmax measurements for each target lesion on 68Ga-DOTATATE and 18F-FDG PET, whole-body tumor segmentation was performed using an open-source and Image Biomarker Standardisation Initiative–compliant software platform called LIFEx platform version 6.3 (24). One radiologist with 8 y of experience performed segmentation on axial PET Digital Imaging and Communications in Medicine images that had been archived in a PACS. The metabolic tumor volume (MTV), and the total lesion glycolysis on 18F-FDG PET and functional tumor volume (FTV) on 68Ga-DOTATATE PET, were semiautomatically delineated using a threshold value of 1.5 times the background liver activity, with minor manual correction to optimize tumor delineation. These parameters were compared for each primary NETPET score and for the groups with low and high NETPET scores.
Statistical Analysis
Descriptive statistics including mean ± SD, frequencies, and percentages were used to summarize patient-level and lesion characteristics, NETPET scores, SUVmax on 68Ga-DOTATATE and 18F-FDG PET, quantitative data, and planned management. Comparison of mean 18F-FDG/68Ga-DOTATATE ratio, FTV for 68Ga-DOTATATE PET, MTV for 18F-FDG PET, and FTV/MTV ratios between NETPET low-score (P1–P2) and high-score (P3–P5) groups was analyzed using the Wilcoxon rank-sum test. The association between lesional SUVmax on 18F-FDG PET and lesional SUVmax on 68Ga-DOTATATE was evaluated using scatterplot analysis with a line of best fit and Pearson correlation coefficient. Statistical analysis was done using R version 4.3.1.
RESULTS
Forty patients were recruited. Table 1 presents their demographic data. Primary tumor sites included gastrointestinal tract (n = 23, including 10 small bowel, 6 colorectal, 4 gastric, and 3 unknown primary tumors) and pancreas (n = 17). On a patient level, primary NETPET scores of P0, P1, P2A, P2B, P3B, P4B, and P5 were documented in 2 (5%), 5 (12.5%), 5 (12.5%) 20 (50%), 2 (5%), 4 (10%), and 2 (5%) patients, respectively. Referral was for initial staging of a GEP NET in 26 patients, of whom 18 (59.2%) had a low NETPET score. Referral was for restaging after prior therapy in 14 patients, all of whom had a low NETPET score. Consensus was reached in all cases, with no need for arbitration by a third reviewer. Overall, a low NETPET score was recorded for 30 of 38 (79%) patients (Fig. 3), and a high score was recorded for 8 of 38 (21%) patients (Fig. 4). In total, 146 target lesions were assessed in the 40 patients. These lesions were in the liver (47/146; 32.2%), lymph nodes (34/146; 23.3%), bone (32/146; 21.9%), pancreas (13/146; 8.9%), peritoneum (7/146; 4.8%), bowel (3/146; 2.1%), lung (3/146; 2.1%), and other soft tissues (7/146; 4.8%). The distribution of target lesion sites was similar for patients with low and high NETPET scores, with more than three quarters of the lesions being in the liver, lymph nodes, or bone (Supplemental Table 1; supplemental materials are available at http://jnm.snmjournals.org).
Demographic Data
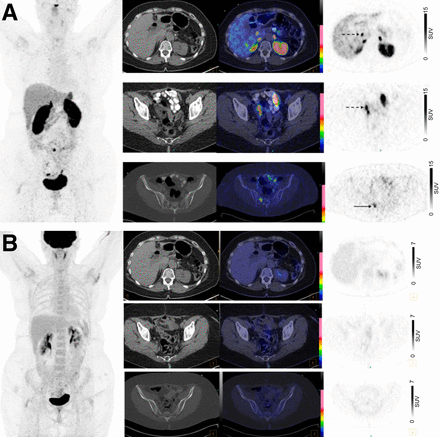
A 62-y-old woman with history of WD grade 2 small-bowel NET on restaging, with NETPET score of 1. (A) 68Ga-DOTATATE PET/CT maximum-intensity projection image (left) and axial 68Ga-DOTATATE CT, fused, and PET images (from left to right) show intensely 68Ga-DOTATATE–avid peritoneal metastases at liver hilum (dotted arrow, top row) and along right pelvic sidewall (dotted arrow, middle row) and 68Ga-DOTATATE–avid sacral bone deposit (solid arrow, bottom row). (B) Corresponding 18F-FDG PET/CT images show no discernible radiotracer uptake at sites of metastatic disease.
A 44-y-old man with history of metastatic WD grade 3 NET (Ki 67, 28%), involving lung, liver, and bone, with unknown primary tumor site and NETPET score of 4B. (A) 18F-FDG PET/CT maximum-intensity projection image (left) and axial (right top: fused and PET from left to right) and coronal 18F-FDG PET/CT images (right bottom: CT, fused, and PET from left to right) show numerous target osteolytic skeletal deposits with intense 18F-FDG uptake (SUVmax, 11.1 in sternum). (B) Corresponding 68Ga-DOTATATE PET/CT maximum-intensity projection image (left) and axial (right top: fused and PET from left to right) and coronal (right bottom: CT, fused, and PET from left to right) 68Ga-DOTATATE PET/CT images show low-level radiotracer uptake in target lesion in sternum (arrow, top) and in right fifth rib (arrow, bottom), with SUVmax of 6.0 and Krenning score of 2.
Quantitative Measures for Target Lesions
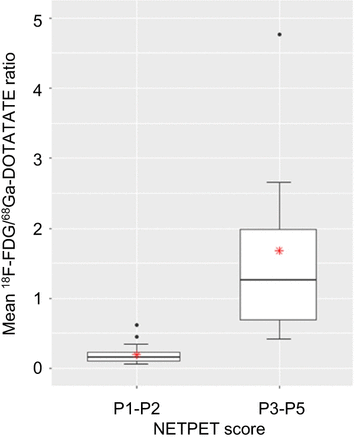
No association was found between the SUVmax of target lesions on 68Ga-DOTATATE PET and 18F-FDG PET, with an SUVmax on 68Ga-DOTATATE and 18F-FDG PET of 31.0 ± 22.6 (range, 1.2–122.1) and 6.1 ± 4.2 (range, 0.7–21.7), respectively (r = −0.06, P = 0.505; Fig. 5). Lesional NETPET scores, and SUVmax on 68Ga-DOTATATE and 18F-FDG PET, are summarized in Table 2. There was no correlation between the SUV of lesions on 68Ga-DOTATATE and 18F-FDG PET (P = 0.505). The 18F-FDG/68Ga-DOTATATE ratios for NETPET scores P1, P2A, P2B, P3B, P4B, and P5 were 0.12 ± 0.06, 0.15 ± 0.09, 0.21 ± 0.16, 0.68 ± 0.17, 2.05 ± 1.88, and 1.26 ± 0.58, respectively. The 18F-FDG/68Ga-DOTATATE ratio was significantly lower for low primary NETPET scores (mean ± SD, 0.20 ± 0.13) than for high primary NETPET scores (mean ± SD, 1.68 ± 1.44) (P < 0.001; Fig. 6).
Mean 18F-FDG to 68Ga-DOTATATE ratio for patients with low NETPET score and those with high NETPET score.
Target Lesion Level Distribution of NETPET Scores
Association between lesional SUVmax on 68Ga-DOTATATE and 18F-FDG PET.
FTV for 68Ga-DOTATATE PET and MTV for 18F-FDG PET for patients by primary NETPET scores are summarized in Table 3. There was no association between FTV on 68Ga-DOTATATE PET and low or high NETPET scores (700 ± 13,016 cm3 and 960 ± 1,230 cm3, respectively; P = 0.75); however, a significantly lower MTV was observed on 18F-FDG PET for primary low NETPET scores compared with high scores (66 ± 114 cm3, 464 ± 601 cm3, and 66 ± 114 cm3, respectively; P = 0.005). Similarly, lower total lesion glycolysis was recorded for low primary NETPET scores than for high scores (286 ± 475 and 3,024 ± 4,631, respectively; P = 0.005). FTV/MTV ratios were significantly lower for high primary NETPET scores than for low scores (2 ± 3 and 42 ± 138, respectively; P < =0.013).
Distribution of FTV and MTV by Primary NETPET Score
Change in Planned Management
Management was grouped by type: curative-intent surgery, palliative-intent surgery (debulking), systemic therapies (somatostatin analogs, chemotherapy, targeted agents, other systemic therapy), liver-directed therapy (embolization), and PRRT. Migration after 18F-FDG PET between types of management was recorded in 17 of 40 patients (42.5%) (Table 4). The most common changes were from systemic therapy to PRRT (all in patients with P1 or P2A/B tumors) and from planned debulking surgery to systemic therapy. For patients with a high NETPET score, a change in type of management was recorded in 7 of 8 (87.5%), compared with 10 of 22 (45.5%) for those with a low NETPET score. Among those who received systemic therapy after 18F-FDG PET, the type of systemic therapy changed in 12 of 18 (66.7%). For patients for whom surgery was planned before 18F-FDG PET (2 with curative-intent surgery and 9 with palliative-intent debulking), only 4 of 11 (36.4%) had surgery as their treatment plan after 18F-FDG PET, whereas all others received systemic therapies. Before 18F-FDG PET, the planned treatment intent was curative, observational, and palliative in 3, 1, and 36 patients, respectively. After PET, 2 of 40 (5%) patients had a change in treatment intent (including 1 patient from curative to palliative and 1 patient from palliative to observational).
Pre– and Post–18F-FDG PET Patient Management Plan
DISCUSSION
WD grade 2 and 3 GEP NETs are heterogeneous in terms of somatostatin receptor expression and tumor metabolic activity, with more than 1 in 5 patients in the current cohort having a high NETPET score, indicating tumors with metabolic activity equal to or above the expression of SSTR-2 on PET. Generally, tumor metabolic activity increases with histologic grade. High tumor metabolic activity is associated with poor prognosis and poor response to PRRT (9–13). Although NETPET scores are derived visually, quantitative measures from dual-tracer PET can differentiate between patients with high and low NETPET scores. Specifically, 68Ga-DOTATATE and 18F-FDG uptake as depicted by 18F-FDG/68Ga-DOTATATE SUVmax ratios were able to discriminate between patients with low and high NETPET scores (ratios of 0.2 ± 0.13 and 1.68 ± 1.44, respectively). Although no association was found between FTV on 68Ga-DOTATATE PET and groups of NETPET scores, there were significantly higher MTVs on 18F-FDG PET and lower FTV/MTV ratios for patients with a high NETPET score than for patients with a low NETPET score.
It has been previously shown that 68Ga-DOTATATE PET changes management in approximately 50% of patients with WD NETs (25). A recent survey performed among experts attending the European Neuroendocrine Tumor Society advisory board meeting in 2022 sought to describe the real-life use of 18F-FDG PET/CT in this patient population. The survey showed high agreement among experts on the use of 18F-FDG PET in patients with a mismatch between conventional imaging and 68Ga-DOTATATE PET, especially in patients being considered for PRRT, and for patients with grade 3 tumors being considered for surgery. Consensus was low for other indications assessed (26). A prior prospective study including 156 patients showed that the 18F-FDG positivity rate increased from 37% in grade 1 to 58% in grade 2 and 94% in grade 3 tumors (10). However, metabolic activity within a tumor may vary with the degree and extent of metabolic activity depending on several factors including tumor grade, proliferation index, tumor size, and tumor hypoxia (27). The phenotypic heterogeneity of WD tumors makes management decisions based on dual-tracer PET complex. A prior prospective study including 104 patients who underwent dual-tracer PET showed that management changes were based on 18F-FDG PET findings in 22 of 104 (21%) patients (18). When grade 1 tumors were excluded, 21 of 68 (31%) patients with grade 2 and 3 tumors had management changes based on 18F-FDG PET. Our results, which were derived from a cohort of grade 2 and 3 WD GEP NETs, are in line with these findings showing a change in the category of management (e.g., surgery, systemic therapy, or PRRT) in over 40% of patients, with the most common changes being from systemic therapy to PRRT and from planned debulking surgery to systemic therapy. These findings suggest a significant impact of dual-tracer PET on patient treatment options and use of health care resources, including avoidance of unwarranted surgeries. Our study findings likely underestimate the impact of 18F-FDG PET on the management of these patients, as there were also changes recorded in the type of systemic therapy given in over two thirds of patients for whom systemic therapy was planned after 18F-FDG PET. The high rate of management change reported in our cohort in comparison to the study reported by Panagiotidis et al. may be due to the differences in patient populations, with the prior study including NETs from multiple primary tumor sites (18).
The results from this study suggest that dual-tracer PET in WD grade 2 and 3 GEP NETs results in a high rate of management type change. The translation of dual-PET–directed management to patient outcomes should be assessed in future research, to further support the use of dual-tracer PET in these patients. Furthermore, correlations between 68Ga-DOTATATE/18F-FDG metrics at a lesion level and patient level (FTV, MTV) and tumor biology, and response to systemic therapies including response to PRRT, are needed.
This study had a few limitations. First, the number of patients was relatively small. However, our population was targeted, including only GEP NETs and only patients with grade 2 and 3 tumors, for which the impact of 18F-FDG PET has been suggested to be high. Future studies on specific patient populations would be needed to further assess the impact of dual-tracer PET in various clinical scenarios (e.g., before surgery). Second, NETPET scores are assessed visually and therefore may be prone to interobserver variability. To minimize the potential impact of this variability, we used the consensus of 2 independent readers. Third, for correlation with quantitative measures, certain NETPET score groups had a limited number of observations. To mitigate this, we grouped NETPET scoring groups to low and high NETPET scores, using 18F-FDG uptake equal to or above DOTATATE uptake to discriminate between the two. A change in planned management was recorded in nearly all patients with a high NETPET score (7/8; 87.5%) and in less than half of those with a low NETPET score (10/22; 45.5%).
CONCLUSION
In patients with WD grade 2 and 3 GEP NETs, NETPET scores are heterogeneously distributed, with more than 1 in 5 patients having a high NETPET score. Quantitative parameters including 18F-FDG/68Ga-DOTATATE SUVmax ratios in target lesions and FTV/MTV ratios can discriminate between patients with high and low NETPET scores. A change in type of management was observed in 42.5% of patients after 18F-FDG PET, with the most common changes being a change from systemic therapy to PRRT and a change from debulking surgery to systemic therapy. Further prospective randomized data are warranted to assess the impact on patient outcomes.
DISCLOSURE
Funding was provided by a 2019 grant from the Canadian Neuroendocrine Tumor Society (CNETS). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What is the distribution of NETPET scores in WD grade 2 and 3 GEP NETs, and what is the impact of the addition of 18F-FDG PET after 68Ga-DOTATATE PET in these patients?
PERTINENT FINDINGS: In a prospective cohort study including 40 patients with WD grade 2 and 3 GEP NETs, 20% of patients were assigned a high NETPET score, and a change in type of management was recorded in 42.5% of patients after 18F-FDG PET.
IMPLICATIONS FOR PATIENT CARE: In patients with WD grade 2 and 3 GEP NETs, dual-tracer (68Ga-DOTATATE and 18F-FDG) PET frequently impacts patient management.
Footnotes
Published online Sep. 12, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication April 22, 2024.
- Accepted for publication August 2, 2024.