Visual Abstract
Abstract
This study evaluates the diagnostic utility of PET/MRI for primary, locoregional, and nodal head and neck squamous cell carcinoma (HNSCC) through systematic review and metaanalysis. Methods: A systematic search was conducted using PubMed and Scopus to identify studies on the diagnostic accuracy of PET/MRI for HNSCC. The search included specific terms and excluded nonhybrid PET/MRI studies, and those with a sample size of fewer than 10 patients were excluded. Results: In total, 15 studies encompassing 638 patients were found addressing the diagnostic test accuracy for PET/MRI within the chosen subject domain. Squamous cell carcinoma of the nasopharynx was the most observed HNSCC subtype (n = 198). The metaanalysis included 12 studies, with pooled sensitivity and specificity values of 93% and 95% per patient for primary disease evaluation, 93% and 96% for locoregional evaluation, and 89% and 98% per lesion for nodal disease detection, respectively. An examination of a subset of studies comparing PET/MRI against PET/CT or MRI alone for evaluating nodal and locoregional HNSCC found that PET/MRI may offer slightly higher accuracy than other modalities. However, this difference was not statistically significant. Conclusion: PET/MRI has excellent potential for identifying primary, locoregional, and nodal HNSCC.
Head and neck squamous cell carcinoma (HNSCC) accounts for 3% of all cancers in the United States, leading to approximately 11,000 deaths annually (1). The burden of HNSCC varies geographically and is predominantly linked to exposure to tobacco-derived carcinogens, excessive alcohol consumption, or both (2). Its incidence and mortality have been rising, particularly because of increased incidences of oropharyngeal cancer (3). The factors correlating with poor outcome in HNSCC include lymph node metastasis, distant metastasis, and advanced disease (4).
[18F]FDG PET/CT imaging has become widely used for detecting and evaluating HNSCC, providing reliable evaluation of nodal and metastatic disease (5–7). This diagnostic modality provides a reliable method for evaluating nodal and metastatic disease. However, it lacks information on primary tumor assessment because of the complex nature of this anatomic area (8). This hybrid imaging technique often fails to detect small lesions, perilesional invasion, or micrometastatic disease sites, which are critical for accurate staging (9). As a result, MRI is considered superior to [18F]FDG PET/CT in evaluating primary tumors (10).
The use of reliable imaging techniques significantly impacts clinical diagnosis and prognosis, with early and accurate disease detection and evaluation enhancing the 5-y survival rate, and therapeutic adherence and advancements also contribute to improved patient outcomes (11). Many oncology institutions use MRI for evaluating local disease and [18F]FDG PET/CT for assessing nodal and metastatic diseases (12). The increasing availability of PET/MRI may eliminate the need for using 2 imaging modalities for comprehensive assessment (12). This study aims to evaluate the current diagnostic efficacy of PET/MRI in primary and nodal disease evaluation of HNSCC.
MATERIALS AND METHODS
Systematic Search Strategy
PubMed and Scopus databases were investigated to extract pertinent research articles. The search was specifically confined to studies that focused on human subjects. We used specific keywords to facilitate the search process (Supplemental Table 1; supplemental materials are available at http://jnm.snmjournals.org). The final search took place on December 21, 2023, without any specific date restrictions. The adopted review protocol has been officially registered on the Prospective Register of Systematic Reviews website (registration ID CRD42024496416).
Inclusion Criteria
A review of English-language studies involving at least 10 patients assessed with PET/MRI for primary or nodal disease in HNSCC focused on diagnostic accuracy. Included studies allowed for contingency table construction, with definitive diagnoses confirmed by histology, radiologic surveillance, or clinical evaluation (Fig. 1A).
(A) Flowchart illustrating criteria for selecting studies to be included in analysis. (B) Summary charts demonstrating results for assessment of risk of bias and applicability concerns for included studies using QUADAS-2 criteria. End results for each domain are expressed as percentages, whereas number of studies is indicated within bars.
Data Extraction
Data from the studies were systematically collected, including primary author, publication year, radiotracer used, patient cohort size, and diagnostic outcomes. Per-patient data analyzed primary HNSCC, whereas per-lesion data focused on nodal HNSCC, leading to calculated pooled sensitivity and specificity for locoregional HNSCC. Locoregional HNSCC pertains to the presence of HNSCC within the primary tumor site, with or without lymph node involvement.
Study Quality Assessment
The Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) criteria were used to screen and evaluate the quality of all the studies included in the analysis. This approach was adopted to guarantee a high-quality evaluation (13). Any debate between 2 reviewers was resolved by consensus (13). The results for risk of bias and applicability were assessed for patient selection, index tests, and reference standard, whereas the flow and timing domains were solely used to evaluate the risk of bias (Fig. 1B).
Statistical Analysis
We evaluated PET/MRI data for diagnosing primary, locoregional, and nodal HNSCC and for calculating sensitivity, specificity, and 95% CIs. A random-effects metaanalysis assessed the overall diagnostic accuracy, using summary receiver-operating-characteristic curves to determine the area under the curve. Heterogeneity was measured by the I2 test, with a result of over 75% indicating substantial inconsistency (14). A metaregression analysis was conducted to assess the potential sources of heterogeneity. The covariates examined encompass the type of HNSCC cancers, the publication year, the study design, the PET/MRI acquisition method, the MRI technique used, and the index test methodology. Publication bias was assessed through visual evaluation of a Deeks funnel plot, and its statistical significance was tested using an Egger test. A significance level of less than 0.05 for each of the implemented hypotheses was used to determine the statistical significance in this analysis (Supplemental Table 2). All statistical analyses were performed using Stata software version 17.
RESULTS
The study identified 145 research articles through electronic database searches, removed 80 duplicates, and excluded 49 on the basis of title and abstract screening, resulting in 16 relevant articles for full-text review. After this review, it was determined that only 15 articles aligned with the topic of interest and were therefore eligible for inclusion in the systematic review. The quality of these studies was evaluated using the QUADAS-2 criteria (Fig. 1B). A detailed elaboration of QUADAS-2 evaluation is represented in Supplemental Table 3.
Systematic Review
This systematic review analyzed studies published between 2011 and 2023, involving 638 patients. Eight studies were prospective (15–22) and 7 were retrospective (23–29), with most conducted in Europe (15–18,24,25,28,29). The primary focus of the included studies was to assess the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of PET/MRI. Some studies also aimed to compare the diagnostic performance of PET/MRI with PET/CT (17,19,21,27–29). A limited number of studies compared PET/MRI with neck MRI (19,21,23,27,29). Most studies used simultaneous PET/MRI scanners (15–18,20,22–24,26,28,29), with other studies adopting retrospective PET/MRI fusion (19,21,25,27). Nine of 15 studies favored PET/MRI over other comparators (15,19–21,23–25,27,28), whereas 4 concluded indifferences (16,17,22,29). Two studies did not include comparators (18,26). All studies used [18F]FDG as the primary radiotracer (Table 1). A more detailed review of included studies is provided in Supplemental Table 4.
Summary of Study Characteristics
Metaanalysis
Of the 15 studies that were included in the systematic review, only 12 met the criteria for further metaanalysis (16–18,20,22–29). Two studies were primarily concerned with investigating the diagnostic effectiveness of PET/MRI in primary tumor local invasion for 2 different subtypes of HNSSC, and thus, they were not included in the metaanalysis (19,21). Furthermore, an additional study lacking the essential datasets required for constructing a 2 × 2 contingency table were also excluded (15).
Diagnostic Test Accuracy of PET/MRI
In terms of patient-based analysis, PET/MRI demonstrated pooled sensitivity and specificity of 93% and 95% for primary HNSCC, respectively. Additionally, PET/MRI exhibited pooled sensitivities of 93% and 89%, respectively, for locoregional and nodal HNSCC. On the other hand, their pooled specificities for locoregional and nodal HNSCC were 96% and 98%, respectively. A significant heterogeneity was observed for nodal and locoregional domains, with I2 ranging from 78% to 98% (Table 2).
Summary of Pooled Sensitivity and Specificity, Positive Likelihood Ratio, Negative Likelihood Ratio, Diagnostic Odds Ratio, and Study Heterogeneity
To provide a global measure of diagnostic accuracy, summary receiver-operating-characteristic curves were rendered to examine the area under the curve. Excellent diagnostic accuracy results have been demonstrated, as reflected by high area-under-the-curve values exceeding 97% in the primary tumor (Fig. 2A), nodal regions (Fig. 2B), and locoregional HNSCC (Fig. 2C).
Summary receiver-operating-characteristic (SROC) curves for PET/MRI in evaluation of primary tumor (A), nodal regions (B), and locoregional HNSCC (C). SENS = sensitivity; SPEC = specificity; AUC = area under curve.
Metaregression
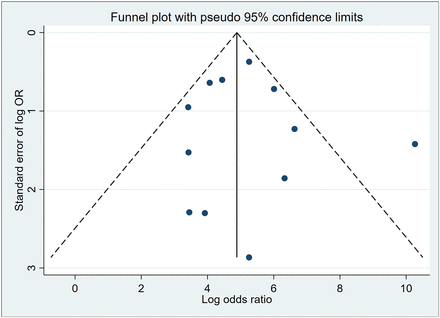
A metaregression analysis explored factors contributing to heterogeneity in HNSCC evaluation using PET/MRI, including cancer type, study design, acquisition method, MRI technique, QUADAS-2 risk, and index test methodology. Study subjects with single HNSCC subtypes had increased sensitivity of PET/MRI up to 100% (P = 0.00). It is worth mentioning that 2 studies specifically focused on enrolling patients with a single HNSCC subtype, namely nasopharyngeal carcinoma (20,26). In addition, MRI acquisition methods adopted without diffusion weight imaging have resulted in a statistically significant specificity improvement (P = 0.03; Table 3). The evaluation of publication bias for PET/MRI in locoregional HNSCC yielded insignificant results (P = 0.87) (Fig. 3).
Results of Metaregression Analysis of PET/MRI for Diagnosis of Locoregional HNSCC
Deeks funnel plot showing relatively evenly distributed studies for locoregional HNSCC evaluated by PET/MRI. OR = odds ratio.
Locoregional HNSCC: Comparative Studies Featuring Other Modalities
In the subgroup analysis of the 5 studies that directly compared locoregional HNSCC using PET/CT versus PET/MRI (17,20,27–29), the pooled sensitivity and specificity of PET/MRI were 95% and 97%, respectively, and the pooled sensitivity and specificity for PET/CT were 86% and 94%, respectively. In this subgroup analysis, though the pooled sensitivity and specificity were higher for PET/MRI than for PET/CT, this difference was not significant (P > 0.25 for each).
Both MRI and PET/MRI exhibit a similar pooled specificity of 98% (P = 0.85) in the subgroup analyses of 4 studies comparing the 2 modalities in locoregional HNSCC (16,17,20,29). Although PET/MRI demonstrated a higher pooled sensitivity of 93% compared with 82% for MRI alone, this difference did not reach statistical significance (P = 0.11).
Nodal HNSCC: Comparative Studies Featuring Other Modalities
Three studies used lesion-based analysis to compare the diagnostic accuracy of [18F]FDG PET/MRI and MRI in assessing nodal HNSCC (16,20,29). The findings indicate that [18F]FDG PET/MRI shows specificity comparable to that of MRI alone (99%; P = 0.31) and a slightly higher sensitivity, although not statistically significant (95% for [18F]FDG PET/MRI vs. 86% for MRI alone; P = 0.16).
In the subgroup analysis of 2 studies comparing nodal HNSCC using [18F]FDG PET/CT versus [18F]FDG PET/MRI (20,29), the cumulative sensitivity and specificity of [18F]FDG PET/MRI were 98% and 99%, respectively, and the cumulative sensitivity and specificity for [18F]FDG PET/CT were 87% and 97%, respectively. The observed differences in pooled specificities did not achieve statistical significance (P > 0.05 for each).
Elaboration on subgroup metaanalysis for nodal and locoregional HNSCC is detailed in Table 4.
Subgroup Analysis of Comparative Studies Featuring Other Modalities
DISCUSSION
Accurate assessment of HNSCC is of paramount importance, as it allows for the implementation of an ideal treatment strategy (30). To assess this, we investigated the diagnostic capabilities of [18F]FDG PET/MRI in primary tumor, nodal, and locoregional HNSCC. Our findings demonstrate that [18F]FDG PET/MRI exhibits excellent diagnostic accuracy, with a per-patient metaanalysis showing a sensitivity of 93% and a specificity of 95% in evaluating HNSCC primary tumors. Additionally, a per-lesion metaanalysis revealed a sensitivity of 89% and a specificity of 98% for nodal HNSCC. Overall, when locoregional HNSCC was assessed, the pooled sensitivity and specificity were 93% and 96%, respectively. The positive likelihood ratios obtained in this study for all evaluated sites were sufficiently high (exceeding 21 for each domain) to detect the involvement of HNSCC, whereas negative likelihood ratio values were optimally low for ruling out HNSCC (≤0.11 for each). The subgroup metaanalysis revealed that [18F]FDG PET/MRI is a robust alternative to [18F]FDG PET/CT and MRI, particularly excelling in nodal and locoregional staging of HNSCC. Although [18F]FDG PET/MRI showed a marginal increase in diagnostic accuracy compared with other methods, this improvement was minimal and not statistically significant, as confirmed by limited comparative studies.
In general, PET/MRI offers valuable anatomic and functional insights, especially in head and neck imaging. It is safer for pediatric patients and those requiring repeated scans as it avoids ionizing radiation from CT (31). However, PET/MRI faces challenges such as longer scan times, higher costs, and limitations in imaging low-proton-density areas such as cortical bone and lungs (32). It is also prone to various artifacts, including those caused by patient movement and metallic implants, which can affect diagnostic outcomes (33). Nevertheless, PET/MRI is less impacted by dental hardware artifacts than PET/CT. Further research is needed to assess its impact on patient outcomes in nuclear oncology, considering recent updates in cancer staging protocols implemented by the most recent American Joint Committee on Cancer (34).
In comparison to other metaanalyses examining the accuracy of [18F]FDG PET/CT for HNSCC, our findings show higher rates than those reported in the most recent metaanalysis (35). In a study by Rohde et al., the combined sensitivity and specificity of [18F]FDG PET/CT in assessing locoregional HNSCC were reported as 89.3% and 89.5%, respectively (35). This metaanalysis also aimed to compare the diagnostic effectiveness of [18F]FDG PET/CT with conventional imaging techniques, and it demonstrated superior outcomes for [18F]FDG PET/CT when compared with other modalities (35). Nevertheless, the authors emphasize the need for further research to improve the diagnostic evaluation of HNSCC because of the presence of considerable false results.
Previously, a comparative analysis of CT, MRI, [18F]FDG PET/CT, and [18F]FDG PET/MRI in advanced buccal squamous cell carcinoma demonstrated that [18F]FDG PET/MRI displayed superior sensitivity and specificity among the 4 modalities (21). Likewise, in the context of HNSCC, Park et al. concluded that [18F]FDG PET/MRI fusion exhibited higher sensitivity and specificity for tumor staging than did MRI and [18F]FDG PET alone (23).
According to our metaregression analysis, MRI acquisition methods adopted without diffusion-weighted imaging have resulted in substantially improved diagnostic accuracy. This can be best ascribed to motion artifacts and oversensitivity to water molecules imposed by diffusion-weighted imaging (36). Another factor of considerable interest is the methodologic variation between retrospective and simultaneous [18F]FDG PET/MRI fusion. Unlike simultaneous techniques, retrospective fusion can cause temporal misalignment and attenuation correction issues (37). Therefore, future clinical investigations should cover and examine these aspects to reach more conclusive results.
The main advantage of adopting PET/MRI comes from its enhanced soft-tissue contrast provided by MRI in these targeted anatomic regions. MRI serves as a precise tool for evaluating the local extent of a tumor, which is crucial in delineating the optimal extent of tumor resection and subsequent therapeutic interventions. However, relying solely on MRI may preclude discerning between benign and malignant alterations in previously operated areas because of factors such as scar tissue, asymmetry loss, and side shift (38). Presently, PET scans offer supplementary insights into tissue metabolic activity (39). Therefore, enhancing diagnostic reliability requires a collective approach. This is supported by one of the included studies, which found that analyzing MRI and PET/CT images concurrently improves the sensitivity (25).
Concerning the N-staging domain, existing research is divergent. Notably, some included studies indicated [18F]FDG PET/MRI superiority over comparators, whereas others do not substantiate this observation (17,27,28). Nevertheless, the scarcity of the currently included studies hinders the execution of a metaregression analysis. Therefore, further prospective comparative studies are needed.
This study has limitations, including the lack of a standardized scanning modality and protocol and the presence of significant heterogeneity within the examined datasets. The study also revealed a subset of 3 outliers in the analysis for nodal disease (23–25). These aforementioned studies share commonalities in their methodology, including different subtypes of HNSCC, a retrospective approach, and a limited patient cohort.
Furthermore, it is essential to recognize 2 preceding metaanalyses published before May 2021, bearing a resemblance in their title to our study (40,41). However, discrepancies in adopted keywords, distinct objectives, and omission of metaregression (Supplemental Table 5) characterize them differently (40,41).
CONCLUSION
Our metaanalysis findings underscore the remarkable diagnostic efficacy of PET/MRI in evaluating primary, locoregional, and nodal HNSCC. In the present circumstances, the absence of high literature render and histopathologic heterogeneity pose significant limitations. Consequently, further prospective investigations and comparative studies are needed to validate the reliability of this modality.
DISCLOSURE
Ken Herrmann has received consultant fees from numerous companies including Advanced Accelerator Applications, Amgen, Bayer, and Siemens Healthineers, among others. He has obtained research grants from entities such as Advanced Accelerator Applications, Boston Scientific, and Janssen. Additionally, he holds stock or other ownership interests in companies such as AdvanCell, Aktis Oncology, and SOFIE Biosciences. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What is the diagnostic accuracy of [18F]FDG PET/MRI for detecting primary, nodal, and locoregional HNSCC?
PERTINENT FINDINGS: This systematic review and metaanalysis demonstrated high levels of cumulative accuracy for primary, nodal, and locoregional domains, exceeding 97% for each.
IMPLICATIONS FOR PATIENT CARE: Given its excellent diagnostic performance, PET/MRI should be investigated in future studies to examine its impact on therapy planning and outcome.
Footnotes
Published online Sep. 12, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication May 6, 2024.
- Accepted for publication July 4, 2024.