Visual Abstract
Abstract
Calcific aortic valve disease (CAVD) is a prevailing disease with increasing occurrence and no known medical therapy. Dcbld2−/− mice have a high prevalence of bicuspid aortic valve (BAV), spontaneous aortic valve calcification, and aortic stenosis (AS). 18F-NaF PET/CT can detect the aortic valve calcification process in humans. However, its feasibility in preclinical models of CAVD remains to be determined. Here, we sought to validate 18F-NaF PET/CT for tracking murine aortic valve calcification and leveraged it to examine the development of calcification with aging and its interdependence with BAV and AS in Dcbld2−/− mice. Methods: Dcbld2−/− mice at 3–4 mo, 10–16 mo, and 18–24 mo underwent echocardiography, 18F-NaF PET/CT (n = 34, or autoradiography (n = 45)), and tissue analysis. A subset of mice underwent both PET/CT and autoradiography (n = 12). The aortic valve signal was quantified as SUVmax on PET/CT and as percentage injected dose per square centimeter on autoradiography. The valve tissue sections were analyzed by microscopy to identify tricuspid and bicuspid aortic valves. Results: The aortic valve 18F-NaF signal on PET/CT was significantly higher at 18–24 mo (P < 0.0001) and 10–16 mo (P < 0.05) than at 3–4 mo. Additionally, at 18–24 mo BAV had a higher 18F-NaF signal than tricuspid aortic valves (P < 0.05). These findings were confirmed by autoradiography, with BAV having significantly higher 18F-NaF uptake in each age group. A significant correlation between PET and autoradiography data (Pearson r = 0.79, P < 0.01) established the accuracy of PET quantification. The rate of calcification with aging was significantly faster for BAV (P < 0.05). Transaortic valve flow velocity was significantly higher in animals with BAV at all ages. Finally, there was a significant correlation between transaortic valve flow velocity and aortic valve calcification by both PET/CT (r = 0.55, P < 0.001) and autoradiography (r = 0.45, P < 0.01). Conclusion: 18F-NaF PET/CT links valvular calcification to BAV and aging in Dcbld2−/− mice and suggests that AS may promote calcification. In addition to addressing the pathobiology of valvular calcification, 18F-NaF PET/CT may be a valuable tool for evaluation of emerging therapeutic interventions in CAVD.
Calcific aortic valve disease (CAVD) is the most common valvular heart disease, with the prevalence of cases tripling and the total number of deaths doubling over the last 3 decades (1). The hallmark of CAVD is a fibrocalcific remodeling of aortic valve leaflets. Leaflet remodeling can range from mild thickening to severe calcification and may impair the valve function, ultimately leading to hemodynamically significant aortic stenosis (AS) (2). Inflammation, fibrosis, and calcification contribute to this process. Discoidin, CUB and LCCL domain containing 2 (DCBLD2), also known as endothelial and smooth muscle cell–derived neuropilinlike protein, is a transmembrane protein (3) downregulated in the aortic valves of patients undergoing surgery for AS (4). We recently reported that DCBLD2-deficient (Dcbld2−/−) mice develop spontaneous aortic valve leaflet calcification and hemodynamically significant AS and have an approximately 50% prevalence of bicuspid aortic valve (BAV) when examined at the age of 1 y.
The valvular calcification, which is more severe in Dcbld2−/− mice with BAV, closely mimics the human disease and is linked to enhanced bone morphogenic protein signaling when DCBLD2 deficiency and BAV are present in combination (4). Accordingly, the Dcbld2−/− mouse model may be a unique tool for preclinical evaluation of emerging therapeutic interventions in AS.
The severity of AS is typically evaluated with echocardiography (5). However, echocardiography cannot provide reliable information on the biologic processes that are involved in CAVD progression. Several emerging molecular imaging techniques, including 18F-NaF PET, can complement echocardiography in this regard (6,7). 18F-NaF binds to foci of calcification via exchange of the 18F-fluoride ion with the hydroxyl group of hydroxyapatite (8). A proposed binding model of 18F-NaF to vascular calcification suggests that there is higher binding of 18F-NaF to foci of microcalcification than macrocalcification (9). Several recent studies have established the feasibility and functional relevance of 18F-NaF PET/CT in patients with CAVD. As such, aortic valve 18F-NaF uptake may be detected on PET/CT images in a large subset of patients with AS and in many cases colocalizes with CT-detected calcification (6,10). Furthermore, whereas there is no added prognostic value of 18F-NaF PET to CT-detected valvular calcification, the aortic valve 18F-NaF signal on serial PET/CT images is indeed predictive of future progression of calcification and AS (11,12). Accordingly, 18F-NaF PET could be valuable for early assessment of the effect of emerging therapeutic interventions, in both the preclinical and the clinical settings (13). However, the feasibility and performance of 18F-NaF PET/CT in preclinical small-animal models of CAVD remain to be determined. Leveraging the Dcbld2−/− mouse model of CAVD, our goal in this study was to validate 18F-NaF PET/CT as a tool for tracking valvular calcification in the mouse while examining the development of aortic valve calcification with aging and its interdependence with BAV and AS in this model.
MATERIALS AND METHODS
Animals
The generation of Dcbld2−/− mice (C57BL/6 background) was previously reported (14). Sixty-nine 3- to 24-mo-old mice of both sexes (Supplemental Fig. 1; supplemental materials are available at http://jnm.snmjournals.org) were split into 3 age groups and used in these studies, which also included an additional 26-mo-old mouse used in the reproducibility experiment. All animal procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committees of Yale University and the Veterans Affairs Connecticut Health Care System.
18F-NaF PET/CT
Under isoflurane anesthesia, the animals were injected intravenously with 22.2 ± 5.7 MBq of 18F-NaF (SOFIE Biosciences) followed by a CT contrast agent (ExiTron nano 12000 [VISCOVER]; volume, 30 μL + 1 μL/g of body weight). PET and CT were performed using a small-animal dedicated PET/CT scanner (Inveon PET/CT; Siemens). A contrast-enhanced CT scan was acquired immediately before the PET acquisition. CT data were acquired over 120 projections across 3 bed frame positions with 33% overlap and an effective voxel size of 111 μm. A subset of mice (n = 14) underwent unenhanced CT imaging under the same acquisition parameters before the PET image acquisition. Emission data were acquired initially for 30 min starting at 1 h after tracer injection and subsequently for 10 min starting at 80 min after injection. The data were reconstructed in 3 × 10 min (or 1 × 10 min) frames using iterative 3-dimensional ordered-subset expectation maximization (2 iterations) with maximum a priori estimation (25 iterations), ordinary Poisson distribution, and a 0.8-mm target resolution. Reconstruction was done at a voxel size of 0.4 × 0.4 × 0.8 mm with decay, attenuation, scatter, normalization, and randoms corrections. PET images were corrected by the injected dose and body weight for SUV measurement and were coregistered with the contrast-enhanced CT images for anatomic reference using the bone signal as a landmark. Image analysis was done on 3D Slicer software (15). 18F-NaF activity was quantified on the 80- to 90-min frame using a volume of interest drawn on the aortic valve with an average size of 1.3 ± 0.3 mm3. All PET measurements are presented as SUVmax. In a subset of animals (5 that were 3–4 mo old and 7 that were 18–24 mo old), PET scanning was followed by 18F-NaF autoradiography 3 d later, with 1 set collected after 7 d. For the other animals, the aortic valves were typically collected 2 d after the PET scans, with 1 set collected after 6 d. The animals were euthanized under isoflurane anesthesia by removal of the heart. The reproducibility of PET scan measurements was assessed in a group of 3 elderly animals (average age, 24 mo old, with 1 mouse 26 mo old) by performing 18F-NaF PET/CT twice 1 wk apart.
18F-NaF Autoradiography and Biodistribution
Under isoflurane anesthesia, the animals were injected with 21.2 ± 12.8 MBq of 18F-NaF (Cardinal Health). At 1 h after injection, the animals were euthanized under isoflurane anesthesia and the entire aorta, including the aortic valve and carotid arteries, was carefully dissected under a stereoscopic microscope (MZ9.5; Leica). For quantitative autoradiography, the aorta and standards of known activity were exposed on a phosphor screen (MultiSensitive Phosphor Screen; PerkinElmer) and subsequently scanned with a phosphor imager (Typhoon Trio; GE Healthcare Life Sciences). The injected dose was calibrated to a set time to account for decay from time of injection to time of exposure. The 18F-NaF signals in the aortic valve and descending thoracoabdominal aorta were measured on calibrated images using Fiji/ImageJ software (National Institutes of Health), and the results were expressed as percentage injected dose (%ID) per square centimeter. To investigate biodistribution, blood samples collected at 5 min after injection and the blood and various tissues collected at the time of euthanasia were weighed; and their radioactivity was measured by γ-well counting (Wizard2; PerkinElmer). Data were expressed as %ID/g for various tissues or %ID/mL for blood.
Statistics
All data numbering less than 8 in a single set were evaluated using nonparametric tests and are presented as median with 25th and 75th percentiles and interquartile range (IQR). We used the Wilcoxon test to compare 2 related groups, the Mann–Whitney U test for 2 independent groups, and the Kruskal–Wallis test for more than 2 groups, with the Dunn test for multiple comparison. Data were considered significant when the P value was less than 0.05. Data numbering 8 or more in each set that also passed the Anderson–Darling normality test were evaluated using parametric tests and reported as mean ± SD. ANOVA with post hoc Tukey analysis for multiple comparison was used to compare more than 2 groups, and Pearson correlation tested the relation between 2 variables. Linear regression was used to compare the rate of calcification between different valve phenotypes. The significance of the difference in the slopes of the regression lines was calculated using analysis of covariance. All statistical analyses were performed using GraphPad Prism, version 9.2.0 (Dotmatics). To assess reproducibility, the PET data acquired in the same animals 1 wk apart were compared by paired t testing.
We excluded 1 sample from data analysis because of difficulty in identifying the valve phenotype. One PET sample was removed because of failure of CT contrast administration, which prevented proper identification of the aortic valve. Robust regression and outlier-removal method analysis within each age group, with Q = 1%, identified 1 autoradiography sample as an outlier. This sample was subsequently removed from all analyses.
RESULTS
Prevalence of BAV in Dcbld2−/− Mice
We previously reported that nearly half of approximately 1-y-old Dcbld2−/− mice have BAV. To expand the scope of this observation, we assessed the prevalence of BAV in younger and older animals. Morphologic analysis of the aortic valve in these animals showed that BAV can be found across different age groups (Fig. 1), with 8 of 26 mice at 3–4 mo, 11 of 22 mice at 10–16 mo, and 12 of 19 mice at 18–24 mo presenting with BAV. Alizarin red staining could detect aortic valve calcification in 4 of 10 Dcbld2−/− mice in the oldest age group but in only 1 of 11 in the youngest group.
Aortic valve phenotypes in Dcbld2−/− mice. Shown are examples of aortic valve Masson trichrome (left) and alizarin red (right) staining from Dcbld2−/− mice with TAV at 3–4 mo, BAV at 3–4 mo, TAV at 18–24 mo, and BAV at 18–24 mo.
18F-NaF PET/CT of Valvular Calcification
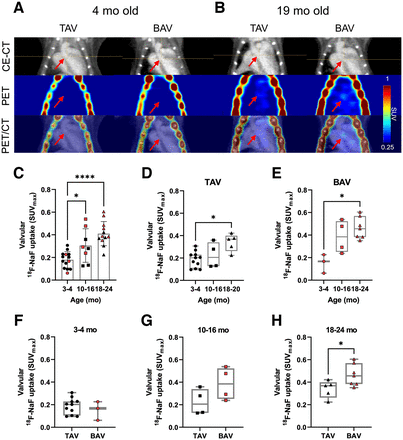
To investigate the timing of aortic valve calcification in this model, Dcbld2−/− mice in 3 different age groups (3–4 mo, n = 14; 10–16 mo, n = 8; and 18–24 mo, n = 12) underwent in vivo 18F-NaF PET/CT (Fig. 2). Qualitative inspection of 18F-NaF PET/CT images showed a distinct aortic valve signal in many animals of the oldest group but rarely in the youngest group (Figs. 2A and 2B). This finding was confirmed on quantitative analysis of the aortic valve 18F-NaF signal on PET/CT images, which showed a significantly higher aortic valve 18F-NaF SUVmax at 18–24 mo (0.41 ± 0.11, P < 0.0001, n = 12) and 10–16 mo (0.31 ± 0.15, P < 0.05, n = 8) than at 3–4 mo (0.18 ± 0.07, n = 14, ANOVA with Tukey post hoc analysis) (Fig. 2C). Histologic evaluation of aortic valves collected after imaging confirmed the presence of both BAV and tricuspid aortic valve (TAV) in each age group, and within each of the TAV and BAV groups, the aortic valve 18F-NaF signal was significantly higher at 18–24 mo than at 3–4 mo (P < 0.05, Kruskal–Wallis test with Dunn post hoc analysis) (Figs. 2D and 2E). A comparison of the aortic valve signal between BAV and TAV animals within each of the 3 age groups showed a trend toward higher valvular 18F-NaF uptake in animals with BAV at 10–16 mo (BAV SUVmax, 0.39 [IQR, 0.25–0.52], vs. TAV SUVmax, 0.21 [IQR, 0.13–0.34]), which reached statistical significance at 18–24 mo (0.46 [IQR, 0.38–0.57] vs. 0.37 [IQR, 0.26–0.4], P < 0.05, Mann–Whitney U test) (Figs. 2F–2H). To assess the reproducibility of the aortic valve signal, a group of animals underwent 2 PET/CT scans within 1 wk. Quantification of the aortic valve 18F-NaF signal showed no significant difference between the 2 scans (scan 1 average SUVmax, 0.30 ± 0.10, vs. scan 2 average SUVmax, 0.28 ± 0.08; P = 0.5; n = 3) with an average of 11% difference between repeat scans (Supplemental Fig. 2).
18F-NaF PET/CT of aortic valve calcification in Dcbld2−/− mice. (A and B) Illustrative coronal images of contrast-enhanced CT, 18F-NaF PET, and PET/CT from 4-mo-old and 19-mo-old Dcbld2−/− mice with TAV or BAV. Arrows point to location of aortic valve. (C) Quantification of aortic valve 18F-NaF signal as SUVmax in different age groups. BAV mice are marked in red. (D and E) Quantification of aortic valve signal as SUVmax in animals with TAV and BAV. (F–H) Quantification of aortic valve signal as SUVmax in mice aged 3–4 mo, 10–16 mo, and 18–24 mo. *P < 0.05. ****P < 0.0001. P values were determined by ANOVA with Tukey multiple comparison post hoc test (C), Kruskal–Wallis with Dunn multiple-comparison post hoc test (D–E), and Mann–Whitney U test (F–H). CE = contrast-enhanced.
18F-NaF Autoradiography
To confirm the effect of aging and BAV on valvular calcification, Dcbld2−/− mice from all 3 age groups underwent autoradiography after in vivo 18F-NaF administration (3–4 mo, n = 17; 10–16 mo, n = 14; 18–24 mo, n = 15; Fig. 3). On visual inspection of images, a distinct 18F-NaF signal was present in BAV samples (Fig. 3A). Quantification of the aortic valve 18F-NaF signal showed a significantly higher tracer uptake in the 18- to 24-mo group (0.07 ± 0.03 %ID/cm2) than in the 10- to 16-mo group (0.04 ± 0.02 %ID/cm2, P < 0.01, ANOVA with Tukey post hoc analysis) and the 3- to 4-mo group (0.04 ± 0.01 %ID/cm2, P < 0.001, ANOVA with Tukey post hoc analysis) (Fig. 3B). Thoracoabdominal aortic uptake was also higher in the 18- to 24-mo group than in the 10- to 16-mo and 3- to 4-mo groups (P < 0.0001, Supplemental Fig. 3A). Next, we compared the aortic valve and aortic signal between BAV and TAV mice across and within different age groups. In TAV mice, the aortic valve 18F-NaF signal was significantly higher at 18–24 mo than at 10–16 mo (0.04 [IQR, 0.03–0.06] vs. 0.02 [IQR, 0.02–0.03] %ID/cm2, P < 0.05, Kruskal–Wallis with Dunn post hoc test) (Fig. 3C). Similarly, in animals with BAV, the aortic valve 18F-NaF signal was significantly higher at 18–24 mo than at 3–4 mo (0.09 [IQR, 0.07–0.11] vs. 0.05 [IQR, 0.04–0.06] %ID/cm2, P < 0.01, Kruskal–Wallis with Dunn post hoc test) (Fig. 3D). Additionally, in the TAV group, the thoracoabdominal aortic uptake was higher at 18–24 mo (0.03 [IQR, 0.02–0.03] %ID/cm2) than at 10–16 mo [0.02 [IQR, 0.02–0.02], P < 0.05, Kruskal–Wallis with Dunn post hoc analysis) and 3–4 mo (0.02 [IQR, 0.01–0.02], P < 0.05, Kruskal–Wallis with Dunn post hoc analysis) (Supplemental Fig. 3B). Similarly, the thoracoabdominal aortic uptake in BAV mice was higher at 18–24 mo (0.03 [IQR, 0.02–0.04] %ID/cm2) than at 10–16 mo (0.02 [IQR, 0.02–0.02] %ID/cm2, P < 0.001, Kruskal–Wallis with Dunn post hoc) (Supplemental Fig. 3C). At all age groups, the animals with BAV had a higher aortic valve 18F-NaF signal than the TAV mice (3–4 mo: 0.05 [IQR, 0.04–0.06] for BAV vs. 0.03 [IQR, 0.02–0.04] for TAV, P < 0.05; 10–16 mo: 0.06 [IQR, 0.04–0.07] for BAV vs. 0.02 [IQR, 0.02–0.03] for TAV, P < 0.01; 18–24 mo: 0.09 [IQR, 0.07–0.11] for BAV vs. 0.04 [IQR, 0.03–0.06] for TAV, P < 0.01, Mann–Whitney U test) (Figs. 3E–3G). However, there was no difference in thoracoabdominal aortic uptake between BAV and TAV mice in older age groups, and only a minor difference (P = 0.046) was present at 3–4 mo (Supplemental Figs. 3D–3F). Importantly, evaluation of 18F-NaF activity in blood at 5 and 60 min after tracer injection did not show any significant difference among the 6 groups of mice (Supplemental Figs. 4A–4B). Finally, there was no significant difference between different groups in 18F-NaF uptake quantified in the heart apex, lung, liver, spleen, kidney, and bone at 60 min after injection (Supplemental Figs. 4C–4H).
Aorta and aortic valve 18F-NaF autoradiography. (A) Examples of 18F-NaF autoradiography in animals with TAV or BAV at 3–4 mo, 10–16 mo, and 18–24 mo. Arrows point to aortic valve. (B) Quantification of aortic valve 18F-NaF uptake across different age groups. BAV mice are marked in red. (C and D) Quantification of aortic valve 18F-NaF uptake in animals with TAV and BAV. (E–G) Quantification of aortic valve signal in mice aged 3–4 mo, 10–16 mo, and 18–24 mo. *P < 0.05. **P < 0.01. ***P < 0.001. P values were determined by ANOVA with Tukey multiple-comparison post hoc test (B), Kruskal–Wallis with Dunn multiple-comparison post hoc test (C and D), and Mann–Whitney U test (E–G). Scale bar = 0.5 cm.
Accuracy of Aortic Valve 18F-NaF PET Signal Quantification
To test the accuracy of aortic valve signal quantification, 18F-NaF PET/CT was followed by autoradiography in a subset of mice (5 at 4 mo and 7 at 19 mo) (Figs. 4A and 4B). Quantification of the aortic valve signal showed a significant correlation between PET and autoradiography signals (Pearson r = 0.79, P < 0.01, Fig. 4C). Notably, the highest 2 samples as measured by both PET/CT and autoradiography were from animals with BAV. A similar significant correlation was present when the average aortic valve PET and autoradiography signals of all 6 groups of animals were compared (r = 0.83, P < 0.05, Fig. 4D). Excluding the 3- to 4-mo-old animals, for which no focal signal could be identified on the PET images, the correlation coefficient in group averages between PET and autoradiography was 0.99 (P < 0.01).
Correlation between aortic valve 18F-NaF signal by PET and autoradiography. (A and B) Illustrative coronal contrast-enhanced CT, 18F-NaF PET, and PET/CT images in 19-mo-old Dcbld2−/− mice with TAV and BAV (A) and their respective aorta and aortic valve autoradiography (B). Arrows point to location of aortic valve. (C and D) Correlation of aortic valve 18F-NaF uptake quantified by PET and autoradiography in same animals (C) and average signal per group (D). Linear regression lines are shown, with 95% confidence interval of line of best fit as dotted lines. SE bars for each group are shown in D. *P < 0.05 and **P < 0.01 for Pearson correlations. Scale bar = 0.5 cm.
Calcification Rate in BAV and TAV
After the observation that aortic valve calcification increased with aging, we sought to determine whether the number of leaflets affects the rate of calcification with aging. There was a significant correlation between calcification as measured by PET and age among both BAV and TAV mice (BAV r = 0.80, P < 0.01; TAV r = 0.61, P < 0.01). Similarly, in both BAV and TAV there was a significant correlation between calcification as measured by autoradiography and age (BAV r = 0.68, P < 0.001; TAV r = 0.40, P < 0.05). Linear regression analysis of PET/CT-determined aortic valve calcification versus the exact animal age showed a significant difference between BAV and TAV (regression slope: 0.009 for TAV [n = 20] vs. 0.021 for BAV [n = 13], P < 0.05) (Fig. 5A). A similar (although not statistically significant) trend between the BAV and TAV calcification rate over time was also detected by autoradiography (regression slope: 0.001 for TAV [n = 25] vs. 0.002 for BAV [n = 21], P = 0.097) (Fig. 5B).
Aortic valve phenotype and calcification rate in Dcbld2−/− mice. Relation between 18F-NaF uptake measured by PET (A) and autoradiography (B), and animal age in mice with TAV and BAV. Linear regression lines are shown, with 95% confidence interval of line of best fit as dotted lines.
Aortic Valve Function and Calcification in Dcbld2−/− Mice
Next, we sought to investigate whether aortic valve calcification and function are linked in any way in Dcbld2−/− mice. Evaluation of transaortic valve flow velocity showed no difference between the animals in different age groups (3–4 mo: n = 26 [BAV, 8]; 10–16 mo: n = 8 [BAV, 4]; 18–24 mo: n = 21 [BAV, 13]). However, within each age group, the animals with BAV had significantly higher transaortic valve flow velocity than the TAV animals (3–4 mo: 1,223 mm/s [IQR, 1,031–1,490 mm/s] for TAV vs. 3,118 mm/s [IQR, 2,387–3,959 mm/s] for BAV, P < 0.0001; 10–16 mo: 1,073 mm/s [IQR, 846–1,580 mm/s] for TAV vs. 3,189 mm/s [IQR, 2,388–4,949 mm/s] for BAV, P < 0.05; 18–24 mo: 1,255 mm/s [IQR, 1,197–1,787 mm/s] for TAV vs. 2,782 mm/s [IQR, 2,048–3,822 mm/s] for BAV, P < 0.001, Mann–Whitney U test) (Figs. 6A–6E). Consistent with these findings, leaflet separation was lower in BAV mice than in TAV mice at 3–4 mo and 18–24 mo of age (P < 0.0001, Supplemental Fig. 5A). In addition, whereas on average there was no significant difference in left ventricular outflow velocity, mass, ejection fraction, or fractional shortening between BAV and TAV in any of the 3 age groups (Supplemental Figs. 5B–5E), in a subset of animals with BAV, aging was associated with a considerable reduction in ejection fraction and increase in left ventricular mass. Finally, in animals that underwent echocardiography before 18F-NaF PET/CT or autoradiography, there was a significant correlation between transaortic valve flow velocity and aortic valve calcification as detected by 18F-NaF PET/CT (r = 0.55, P < 0.001) or autoradiography (r = 0.45, P < 0.01) (Figs. 6F–6G).
Aortic valve echocardiography and its relation to calcification in Dcbld2−/− mice. (A and B) Illustrative screenshots of transaortic valve velocity by Doppler echocardiography in 19-mo-old TAV (A) and BAV (B) mice. BAV y-axis velocity scale is double TAV scale. (C–E) Transaortic valve peak jet velocity of TAV and BAV mice aged 3–4 mo, 10–16 mo, and 18–24 mo. *P < 0.05, Mann–Whitney U test. ***P < 0.001, Mann–Whitney U test. ****P < 0.0001, Mann–Whitney U test. (F and G) Correlation between peak transaortic valve velocity and aortic valve 18F-NaF signal by PET (F) and autoradiography (G). **P < 0.01, Pearson correlation. ***P < 0.001, Pearson correlation.
DISCUSSION
After the feasibility of 18F-NaF PET/CT and autoradiography was established, these molecular imaging techniques were leveraged to examine the development of aortic valve calcification in Dcbld2−/− mice. Our data show that aging and BAV are both associated with aortic valve calcification and that, compared with TAV, BAV is associated with a higher rate of valvular calcification over time. Finally, there was a significant correlation between aortic valve calcification and stenosis in this murine model.
Ectopic calcification can be found in a wide range of cardiovascular diseases, from coronary artery disease to aortic aneurysms and CAVD (16,17). Since 18F-NaF binds to the surface of calcified deposits, the higher surface-to-volume ratio of microcalcifications than of macrocalcifications results in higher 18F-NaF uptake in foci of microcalcification than in macrocalcifications of a similar total volume (9). The size of microcalcifications can be too small to be detectable by CT, which also requires higher calcium densities to yield sufficient attenuation and, thus, contrast between calcium deposits and surrounding tissues. This was indeed the case in our model, where we could not detect any distinct aortic valve calcification on unenhanced CT performed on a subset of animals (data not shown). As such, CT attenuation in vivo (or alizarin red staining ex vivo) may not directly match 18F-NaF binding to different sites of calcification (16). Because small foci of microcalcification can coalesce to form larger calcium deposits, the 18F-NaF signal on PET can correspond to the foci of future CT-detectable calcification (11,16,18). As such, 18F-NaF PET/CT can be used as a tool to assess valve deterioration and track the effect of therapeutic interventions in the clinical setting (12,19,20). Accordingly, 18F-NaF PET/CT is a potential tool for tracking of the calcification process and for early assessment of the effect of therapeutic interventions on ectopic calcification in not only the clinical setting but also the preclinical setting.
Genetically modified murine models are powerful tools to study aortic valve biology and may be of value as a first step in drug development and testing. We recently introduced Dcbld2−/− mice as a new model of CAVD that phenocopies human disease and may therefore be of value for such applications (4). Dcbld2−/− mice show a high prevalence of BAV and valvular calcification, which we have linked to the interplay of leaflet numbers and enhanced bone morphogenic protein 2 signaling due to DCBLD2 deficiency. The limitations of alizarin red staining used in that study motivated us to leverage 18F-NaF imaging to gain a fuller picture of valvular calcification in this model. To date, only a few studies have evaluated and taken advantage of 18F-NaF PET in preclinical studies of cardiovascular disease. These include an evaluation of 18F-NaF binding to atherosclerotic plaque in minipigs (21) and mice (22,23); however, none has focused on CAVD.
The absence of 18F-NaF myocardial uptake facilitates aortic valve imaging. However, PET/CT of the aortic valve in a mouse is challenging because of the small size of the valve (∼1–2 mm) relative to the spatial resolution of small-animal PET (∼1.6 mm) (24) and spillover from thoracic vertebrae near the aortic valve and ascending aorta. These challenges are not present in ex vivo autoradiography, and therefore, we relied on autoradiography to complement in vivo imaging data. Importantly, the positive correlation between in vivo and ex vivo measurements of the 18F-NaF signal within the same animals validated our approach to quantifying the 18F-NaF signal in vivo, justifying further use of 18F-NaF PET/CT to assess and track calcification in small-animal models.
Although we have not detected any clear valvular calcification by CT, even in older animals, both 18F-NaF PET and autoradiography showed an increase in aortic valve microcalcification with aging. This finding indicates that similar to humans, in whom advanced age is a major risk factor for CAVD (25), calcification is an acquired phenotype in Dcbld2−/− mice. Again, as in humans, 18F-NaF uptake increases with aging in Dcbld2−/− mice regardless of the BAV or TAV status. Of note, the aortic valve SUVmax is lower in this mouse model than in humans (6). This lower SUVmax may be related to the partial-volume effect because of the small size of the aortic valve, cardiac motion, and possibly the intensity of calcification in this mouse model compared with humans. Interestingly, the background signal in the mediastinum is increased in older mice despite comparable radioactivity in the blood and most other tissues across all age groups. This increased background signal, along with the increased aortic activity, suggests that the higher mediastinal activity seen with aging could be related to diffuse vascular microcalcification.
Previous work has shown the presence of AS in approximately 1-y-old Dcbld2−/− mice with BAV. Our results indicate that AS persists with aging. Interestingly, AS is also present in younger BAV mice. Overall, despite the increase in calcification, the severity of AS did not increase with aging. Stenotic valves are generally more calcified in humans, and the extent of calcification correlates with stenosis. Indeed, recent guidelines recommend calcium score as a key parameter to differentiate moderate from severe aortic valve stenosis when the distinction is otherwise difficult to establish (26). However, the relation between aortic valve stenosis and calcification is complex, as other processes, such as fibrosis, contribute to valvular dysfunction (27,28). The positive correlation between calcification and stenosis suggests the two are also linked in Dcbld2−/− mice. However, the increase in calcification without any change in stenosis over time suggests that calcification is dependent on stenosis in this model, and the opposite may not be true. Alternatively, calcification and stenosis could both be driven by BAV while remaining independent of each other.
CONCLUSION
18F-NaF PET/CT links aortic valve calcification to BAV and aging in Dcbld2−/− mice and suggests that AS may promote valvular calcification. In addition to its role in addressing the pathobiology of valvular calcification, 18F-NaF PET/CT may serve as a valuable tool for preclinical evaluation of therapeutic interventions aimed at preventing or reducing aortic valve calcification. Given the complementary roles of calcification and fibrosis in AS, 18F-NaF PET/CT could be combined with molecular imaging of fibrosis or extracellular remodeling, for example, through matrix metalloproteinase imaging (29), to provide a comprehensive picture of the disease process in CAVD.
DISCLOSURE
This work was supported by grants from NIH (R01AG065917, R01HL138567, and R01HL161746) and the Department of Veterans Affairs (I0-BX004038). Azmi Ahmad was supported by NIH training grant T32HL098069. Jakub Toczek and Mehran Sadeghi are inventors on a Yale patent: “New Tracers for Matrix Metalloproteinase Imaging.” No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What is the relationship between aortic valve calcification and aging and its interdependence with BAV and AS in a mouse model of CAVD?
PERTINENT FINDINGS: Leveraging the Dcbld2−/− mouse model of CAVD, we demonstrated the feasibility and validity of 18F-NaF PET/CT for detection of aortic valve calcification. 18F-NaF uptake in the aortic valve significantly increased with aging and was significantly higher in animals with BAV than in those with TAV, with multimodality imaging suggesting that AS may promote valvular calcification.
IMPLICATIONS FOR PATIENT CARE: In addition to its role in addressing the pathobiology of valvular calcification, 18F-NaF PET/CT can serve as a valuable tool for preclinical evaluation of therapeutic interventions aimed at preventing or reducing aortic valve calcification.
Footnotes
Published online Jun. 15, 2023.
- © 2023 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication January 26, 2023.
- Revision received April 25, 2023.