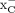Visual Abstract
Abstract
System  is an appealing biomarker for targeting oxidative stress with oncologic PET imaging and can serve as an alternative PET biomarker to other metabolic indicators. In this paper, we report a direct comparison of 2 18F-labeled amino acid radiopharmaceuticals targeting system
is an appealing biomarker for targeting oxidative stress with oncologic PET imaging and can serve as an alternative PET biomarker to other metabolic indicators. In this paper, we report a direct comparison of 2 18F-labeled amino acid radiopharmaceuticals targeting system  , [18F]5-fluoroaminosuberic acid ([18F]FASu) and (4S)-4-(3-[18F]fluoropropyl)-l-glutamate ([18F]FSPG), in terms of their uptake specificity and ability to image glioma and lung cancer xenografts in vivo. Methods: Both tracers were synthesized according to previously published procedures. In vitro uptake specificity assays were conducted using prostate (PC-3), glioblastoma (U-87), colorectal (HT-29), ovarian (SKOV3), breast (MDA-MB-231), and lung cancer (A549) cell lines. PET/CT imaging and biodistribution studies were conducted in immunocompromised mice bearing U-87 or A549 xenografts. Results: In vitro cell uptake assays showed that the tracers accumulated in cancer cells in a time-dependent manner and that the uptake of [18F]FASu was blocked by the system
, [18F]5-fluoroaminosuberic acid ([18F]FASu) and (4S)-4-(3-[18F]fluoropropyl)-l-glutamate ([18F]FSPG), in terms of their uptake specificity and ability to image glioma and lung cancer xenografts in vivo. Methods: Both tracers were synthesized according to previously published procedures. In vitro uptake specificity assays were conducted using prostate (PC-3), glioblastoma (U-87), colorectal (HT-29), ovarian (SKOV3), breast (MDA-MB-231), and lung cancer (A549) cell lines. PET/CT imaging and biodistribution studies were conducted in immunocompromised mice bearing U-87 or A549 xenografts. Results: In vitro cell uptake assays showed that the tracers accumulated in cancer cells in a time-dependent manner and that the uptake of [18F]FASu was blocked by the system  inhibitor sulfasalazine and rose bengal, but not by system L inhibitor 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid, system
inhibitor sulfasalazine and rose bengal, but not by system L inhibitor 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid, system  inhibitor L-trans-pyrrolidine-2,4-dicarboxylic acid, or l-serine, which is a substrate for transporter systems A, ACS, B0, and B0,+. Conversely, [18F]FSPG uptake decreased significantly in the presence of an excess of L-trans-pyrrolidine-2,4-dicarboxylic acid in 2 of 3 tested cell lines, indicating some reliance on system
inhibitor L-trans-pyrrolidine-2,4-dicarboxylic acid, or l-serine, which is a substrate for transporter systems A, ACS, B0, and B0,+. Conversely, [18F]FSPG uptake decreased significantly in the presence of an excess of L-trans-pyrrolidine-2,4-dicarboxylic acid in 2 of 3 tested cell lines, indicating some reliance on system  in these cells. In an in vivo setting, [18F]FASu and [18F]FSPG generated good-contrast PET images in U-87 and A549 tumor–bearing mice. Tracer accumulation in A549 tumors was 5.0 ± 0.8 percentage injected dose (%ID)/g ([18F]FASu, n ≥ 5) and 6.3 ± 1.3 %ID/g ([18F]FSPG, n ≥ 6, P = 0.7786), whereas U-87 xenografts demonstrated uptake of 6.1 ± 2.4 %ID/g ([18F]FASu, n ≥ 4) and 11.2 ± 4.1 %ID/g ([18F]FSPG, n ≥ 4, P = 0.0321) at 1 h after injection. Conclusion: [18F]FSPG had greater in vitro uptake than [18F]FASu in all cell lines tested; however, our results indicate that residual uptake differences exist between [18F]FSPG and [18F]FASu, suggesting alternative transporter activity in the cell lines tested. In vivo studies demonstrated the ability of both [18F]FASu and [18F]FSPG to image glioblastoma (U-87) and non–small cell lung cancer (A549) xenografts.
in these cells. In an in vivo setting, [18F]FASu and [18F]FSPG generated good-contrast PET images in U-87 and A549 tumor–bearing mice. Tracer accumulation in A549 tumors was 5.0 ± 0.8 percentage injected dose (%ID)/g ([18F]FASu, n ≥ 5) and 6.3 ± 1.3 %ID/g ([18F]FSPG, n ≥ 6, P = 0.7786), whereas U-87 xenografts demonstrated uptake of 6.1 ± 2.4 %ID/g ([18F]FASu, n ≥ 4) and 11.2 ± 4.1 %ID/g ([18F]FSPG, n ≥ 4, P = 0.0321) at 1 h after injection. Conclusion: [18F]FSPG had greater in vitro uptake than [18F]FASu in all cell lines tested; however, our results indicate that residual uptake differences exist between [18F]FSPG and [18F]FASu, suggesting alternative transporter activity in the cell lines tested. In vivo studies demonstrated the ability of both [18F]FASu and [18F]FSPG to image glioblastoma (U-87) and non–small cell lung cancer (A549) xenografts.
Oxidative stress (OS), resulting from the imbalance between the production of reactive oxygen species and their elimination by antioxidants (1), has been implicated in the metabolic reprogramming of cancer cells, causing them to become less sensitive to high levels of reactive oxygen species than normal cells (1,2). System  is a transmembrane transporter protein playing a partial role in this process through its action as the primary importer of intracellular cystine (3), which on entry into the cell is reduced to cysteine, the rate-limiting precursor in the biosynthesis of glutathione (2). Glutathione is vital for the maintenance of cellular redox balance and protection from OS (4,5). Consequently, system
is a transmembrane transporter protein playing a partial role in this process through its action as the primary importer of intracellular cystine (3), which on entry into the cell is reduced to cysteine, the rate-limiting precursor in the biosynthesis of glutathione (2). Glutathione is vital for the maintenance of cellular redox balance and protection from OS (4,5). Consequently, system  is upregulated under OS and is found to be overexpressed in several different malignancies, including breast, pancreatic, and brain cancers (6–8). As a result, system
is upregulated under OS and is found to be overexpressed in several different malignancies, including breast, pancreatic, and brain cancers (6–8). As a result, system 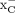 has emerged as a promising target in PET imaging, with several 18F or 11C radiopharmaceuticals reported to target system
has emerged as a promising target in PET imaging, with several 18F or 11C radiopharmaceuticals reported to target system 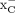 to date (9–14). Among them, [18F]5-fluoro-ASu ([18F]FASu) and (4S)-4-(3-[18F]fluoropropyl)-l-glutamate ([18F]FSPG) are the most studied (Figs. 1A and 1B). [18F]FSPG, a glutamate analog that demonstrated specific tumor uptake, is currently under evaluation in multiple multicenter clinical trials to determine its efficacy in detection and staging of various types of cancer, including, but not limited to, colorectal, breast, pulmonary, abdominal, and head and neck neoplasms (15). [18F]FASu has been used in preclinical evaluations of breast cancer, lung cancer, and glioblastoma (16,17). Both tracers demonstrated the ability to target tumors with good specificity and contrast.
to date (9–14). Among them, [18F]5-fluoro-ASu ([18F]FASu) and (4S)-4-(3-[18F]fluoropropyl)-l-glutamate ([18F]FSPG) are the most studied (Figs. 1A and 1B). [18F]FSPG, a glutamate analog that demonstrated specific tumor uptake, is currently under evaluation in multiple multicenter clinical trials to determine its efficacy in detection and staging of various types of cancer, including, but not limited to, colorectal, breast, pulmonary, abdominal, and head and neck neoplasms (15). [18F]FASu has been used in preclinical evaluations of breast cancer, lung cancer, and glioblastoma (16,17). Both tracers demonstrated the ability to target tumors with good specificity and contrast.
(A) [18F]FASu in vitro uptake at 20, 40, and 60 min in absence and presence of xCT inhibitor sulfasalazine (1 mM). (B) [18F]FSPG in vitro uptake at 20, 40, and 60 min in absence and presence of 1 mM sulfasalazine. Radiotracer structures are embedded into graphs. (C) [18F]FASu and [18F]FSPG in vitro uptake in presence of sulfasalazine (1 mM). Cells were incubated with tracer (red bars for [18F]FASu, black bars for [18F]FSPG) for 20, 40, or 60 min. Sulfasalazine was coadded with tracer. All uptake values are normalized to protein concentration and presented as percentage uptake per minute per milligram of protein (A and B) or as counts per minute per microgram of protein (C). SSZ = sulfasalazine. *P < 0.05. **P < 0.01. ***P < 0.001. ****P < 0.0001.
Herein, we report a comparative preclinical evaluation of 2 system  –imaging agents, [18F]FASu and [18F]FSPG, in tumor accumulation and specificity toward
–imaging agents, [18F]FASu and [18F]FSPG, in tumor accumulation and specificity toward  . The comparison study was performed in non–small cell lung cancer (A549) and glioblastoma (U-87) cell lines and xenograft-bearing mice.
. The comparison study was performed in non–small cell lung cancer (A549) and glioblastoma (U-87) cell lines and xenograft-bearing mice.
MATERIALS AND METHODS
Chemicals and Instrumentation
All chemicals and solvents were obtained from commercial sources and used without further purification. The quantity of injected radiofluorinated tracers was measured using a Capintec CRC-25R/W dose calibrator, and the radioactivity of mouse tissues collected from biodistribution studies was counted using a Perkin Elmer Wizard 2480 γ-counter. PET imaging experiments were conducted using an Inveon multimodality small-animal PET/CT system (Siemens Healthineers).
[18F]FASu Synthesis
[18F]FASu was synthesized as previously reported (18), with a minor change in formulation. The supplemental materials provide further details (available at http://jnm.snmjournals.org). The original [18F]FASu formulation used a trifluoroacetic acetate counterion, but for this study, we used a tracer formulated with a chloride counterion. This change was implemented to match formulations between [18F]FASu and [18F]FSPG. Our validation biodistribution studies indicated no statistically significant differences in biodistribution of [18F]FASu based on the formulation used (Supplemental Fig. 1). Decay-corrected radiochemical yield (d.c. RCY) was 18 ± 6% (n = 6), radiochemical purity was greater than 98%, and molar activity was 17.5 ± 7 GBq/mmol (n = 6).
[18F]FSPG Synthesis
[18F]FSPG was synthesized as previously reported (12). The final product was purified by an SCX cation exchange column and taken up in phosphate-buffered saline buffer. The decay-corrected radiochemical yield was 28 ± 7% (n = 4), radiochemical purity was greater than 98%, and molar activity was 15 ± 5 GBq/mmol (n = 4).
In Vitro Uptake Specificity Studies
All cell lines used in this study were authenticated by DDC Medical.
For tracer uptake and competition studies, the tumor cells were seeded in 24-well plates at appropriate concentrations. The cell number used for seeding was adjusted for every tumor cell line to yield approximately 200,000 cells per well on the day of the uptake study. Cells were usually grown for 2–3 d under standard conditions (37°C, 5% CO2) until subconfluency. The cell number on the day of the uptake assay was determined by detaching cells in 3 representative wells and counting the cells using the MOXI Z mini automated cell counter kit (Orflo). Uptake data were normalized to 100,000 cells or per the protein content in representative wells.
Before the radioactive uptake assay, the cell culture medium was removed, and the cells were washed twice with N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) basal salt solution buffer. Radiotracers were added to the assay buffer using 148 kBq per well. For competition experiments, the cells were coincubated with competitors either in excess at 1 mM or in a dose-dependent manner (0.001–1 mM). Tracer uptake was stopped by removal of the assay buffer at the indicated time points. Cells were quickly washed twice with 400 μL of ice-cold N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) basal salt solution buffer and lysed with the addition of 1 M NaOH. The cell lysate was removed from the plates. Radioactivity of 18F samples was determined using a γ-counter.
Animal Studies
All animal experiments were conducted in accordance with the guidelines established by the Canadian Council on Animal Care and approved by the Animal Ethics Committee of the University of British Columbia. Immunodeficient 129S6/SvEvTac-Rag2tm1Fwa (Rag2M) mice were bred in-house at the Animal Research Centre, British Columbia Cancer Research Institute, and used in this study. Food and water were provided ad libitum for the entire duration of the study.
Tumor Inoculation
Mice were anesthetized briefly with 2.5% isoflurane in oxygen, 2.0 L/min, during cell implantation. After the upper back area below the left shoulder was shaved, the injection site was wiped with an alcohol prep pad, and a 28.5-gauge needle was used to subcutaneously inject approximately 5 × 106 U-87 cells or approximately 2 × 106 A549 cells (in 100 μL 1× phosphate-buffered saline and BD Matrigel Matrix at a 1:1 ratio). Biodistribution studies and PET/CT imaging were performed when tumors reached 5–7 mm in diameter.
Biodistribution Studies
Tumor-bearing mice were briefly anesthetized with isoflurane inhalation and injected with 0.9–2.5 MBq of [18F]FASu or [18F]FSPG (100–200 μL in saline, intravenously). The mice were allowed to roam freely in their cages for 1 h and were then killed by CO2 asphyxiation. Their blood was promptly harvested by cardiac puncture. Organs or tissues of interest were collected in a subsequent necropsy, washed with phosphate-buffered saline, blotted dry, and weighed, and their activity was counted, normalized to the injected dose, and expressed as the percentage injected dose per gram of tissue (%ID/g).
PET Imaging and Data Analysis
Anesthetized mice were injected with 3.94–5.22 MBq through the caudal vein. A 10-min CT scan was performed, followed by a 15-min static or 60-min dynamic PET acquisition on the small-animal PET/CT scanner. PET data were acquired in list mode. After the static acquisition at 1 h after injection, the mice were killed by CO2 asphyxiation, followed by cardiac puncture. The tissues of interest were harvested, weighed, and counted on the γ-counter. The PET data were reconstructed using the 3-dimensional ordered-subset expectation maximization (2 iterations) maximum a priori (18 iterations) algorithm with CT-based attenuation correction. Inveon Research Workplace software (Siemens Healthineers) was used for image analysis and drawing 3-dimensional regions of interest to determine the %ID/g of tissue for selected organs. In addition, Inveon Research Workplace software was used to generate maximum-intensity projection images for visualization.
Statistical Analysis
All data are expressed as mean ± SD. Statistical analysis was performed using GraphPad (7.0-h) software. Two-way ANOVA analysis was performed for all tracer comparisons for in vitro and biodistribution studies. Multiple comparisons were corrected using the Šidak method. Student t tests were performed for all organs and tumor-to-organ ratios in the biodistribution blocking studies. The difference was considered statistically significant at a P value of less than 0.05.
RESULTS
In Vitro Cell Uptake Studies
Uptake of both [18F]FASu and [18F]FSPG was measured in 5 different human cancer cell lines: MDA-MB-231 (breast), U-87 (glioblastoma), HT-29 (colorectal), A549 (lung), and SKOV3 (ovarian). The uptake generally increased over time and was blocked by the system  inhibitor sulfasalazine (1 mM) in all cases (Figs. 1A and 1B). [18F]FSPG had greater uptake overall, with U-87 cells retaining 5.8% ± 0.5% activity/mg of protein at 60 min and A549 and SKOV3 exceeding 29.6% ± 2.4% uptake/mg of protein (Fig. 1B). [18F]FASu uptake was also inhibited by the addition of sulfasalazine in all cell lines studied (Fig. 1A); however, the activity taken up by the cells in the absence of sulfasalazine ranged from 1.8% ± 0.1% uptake/mg of protein to 12.2% ± 1.0% uptake/mg of protein at 60 min in A549 cells. Figure 1C illustrates uptake values in the presence of excess sulfasalazine, where it is evident that the uptake of the 2 tracers no longer differed by severalfold, in all cell lines except SKOV3. Furthermore, we observed that the uptake of [18F]FASu in the presence of 1 mM sulfasalazine did not change with increasing incubation time, except in A549 cells, where it increased from 0.650% ± 0.042% uptake/mg of protein at 20 min to 0.988% ± 0.242% uptake/mg of protein at 60 min (P < 0.0001). Under the same conditions, [18F]FSPG uptake continued increasing with prolonged incubation in all cell lines despite the presence of 1 mM sulfasalazine, with the exception of U-87 cells, which demonstrated no significant change over time (P = 0.7069 for 20- vs. 60-min comparison and P > 0.89 for 20- vs. 40-min and 40- vs. 60-min comparisons; Fig. 1C).
inhibitor sulfasalazine (1 mM) in all cases (Figs. 1A and 1B). [18F]FSPG had greater uptake overall, with U-87 cells retaining 5.8% ± 0.5% activity/mg of protein at 60 min and A549 and SKOV3 exceeding 29.6% ± 2.4% uptake/mg of protein (Fig. 1B). [18F]FASu uptake was also inhibited by the addition of sulfasalazine in all cell lines studied (Fig. 1A); however, the activity taken up by the cells in the absence of sulfasalazine ranged from 1.8% ± 0.1% uptake/mg of protein to 12.2% ± 1.0% uptake/mg of protein at 60 min in A549 cells. Figure 1C illustrates uptake values in the presence of excess sulfasalazine, where it is evident that the uptake of the 2 tracers no longer differed by severalfold, in all cell lines except SKOV3. Furthermore, we observed that the uptake of [18F]FASu in the presence of 1 mM sulfasalazine did not change with increasing incubation time, except in A549 cells, where it increased from 0.650% ± 0.042% uptake/mg of protein at 20 min to 0.988% ± 0.242% uptake/mg of protein at 60 min (P < 0.0001). Under the same conditions, [18F]FSPG uptake continued increasing with prolonged incubation in all cell lines despite the presence of 1 mM sulfasalazine, with the exception of U-87 cells, which demonstrated no significant change over time (P = 0.7069 for 20- vs. 60-min comparison and P > 0.89 for 20- vs. 40-min and 40- vs. 60-min comparisons; Fig. 1C).
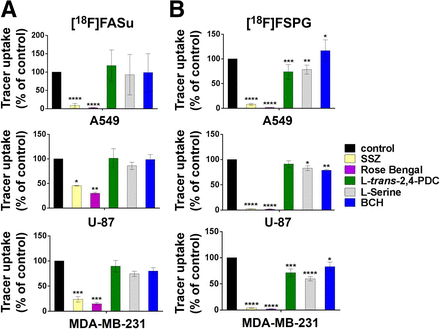
In vitro specificity assays were performed in A549, U-87, and MDA-MB-231 cell lines using the system  inhibitor sulfasalazine and inhibitors of the excitatory amino acid transporter protein (EAAT) family of transporters (L-trans-pyrrolidine-2,4-dicarboxylic acid [PDC]) (19) of vesicular glutamate transporters (rose bengal) (20) and of system L transporter (2-aminobicyclo-[2,2,1]-heptane-2-carboxylic acid) (21–23), in addition to l-serine, which is a known substrate of transporter systems A, ASC, B0, and asc (24). Once again, sulfasalazine significantly inhibited uptake of both [18F]FASu and [18F]FSPG (P < 0.05 for each comparison, n = 3; Fig. 2; Supplemental Fig. 2). [18F]FASu uptake was not significantly inhibited in the presence of excess PDC, l-serine, or 2-aminobicyclo-[2,2,1]-heptane-2-carboxylic acid (P value varied but exceeded 0.463 for each comparison). Conversely, [18F]FSPG uptake decreased significantly in the presence of excess PDC in A549 and MDA-MB-231 cells (P = 0.0006 and P = 0.0002, respectively, equivalent to 26.7% and 28.7% uptake inhibition), but not in the U-87 cell line (P = 0.4570, corresponding to 8.9% uptake inhibition), which indicated possible involvement of EAATs in these cell lines. This finding is consistent with those of Koglin et al. and Greenwood et al., who also reported competition of [18F]FSPG uptake with both aspartate and glutamate, which are the natural substrates of the EAATs (12,24). Moreover, we observed significant uptake blocking in the presence of l-serine in all 3 cell lines (P = 0.0047 for A549, P = 0.0340 for U-87, and P = 0.0001 for MDA-MB-231 cells, equivalent to 21.7%, 17.0%, and 40.2% [18F]FSPG uptake inhibition, respectively). We also found that the most potent vesicular glutamate transporter inhibitor, rose bengal (25), inhibited the uptake of both [18F]FASu and [18F]FSPG (P value varied but was ≤0.0015 for each comparison) and that it had better blocking efficacy than sulfasalazine in the case of both tracers and across all cell lines studied (Fig. 2; Supplemental Fig. 3). Western blotting indicated expression of EAAT3 and EAAT4 transporters in U-87 and MDA-MB-231 whole-cell lysates (Supplemental Fig. 4). Furthermore, blotting of EAAT1 and EAAT2 indicated bands corresponding to the expression of glycosylated proteins, causing a band shift of 5–15 kDa (26), as well as detection of EAAT1 homodimers and homotrimers in all 3 cell lines tested.
inhibitor sulfasalazine and inhibitors of the excitatory amino acid transporter protein (EAAT) family of transporters (L-trans-pyrrolidine-2,4-dicarboxylic acid [PDC]) (19) of vesicular glutamate transporters (rose bengal) (20) and of system L transporter (2-aminobicyclo-[2,2,1]-heptane-2-carboxylic acid) (21–23), in addition to l-serine, which is a known substrate of transporter systems A, ASC, B0, and asc (24). Once again, sulfasalazine significantly inhibited uptake of both [18F]FASu and [18F]FSPG (P < 0.05 for each comparison, n = 3; Fig. 2; Supplemental Fig. 2). [18F]FASu uptake was not significantly inhibited in the presence of excess PDC, l-serine, or 2-aminobicyclo-[2,2,1]-heptane-2-carboxylic acid (P value varied but exceeded 0.463 for each comparison). Conversely, [18F]FSPG uptake decreased significantly in the presence of excess PDC in A549 and MDA-MB-231 cells (P = 0.0006 and P = 0.0002, respectively, equivalent to 26.7% and 28.7% uptake inhibition), but not in the U-87 cell line (P = 0.4570, corresponding to 8.9% uptake inhibition), which indicated possible involvement of EAATs in these cell lines. This finding is consistent with those of Koglin et al. and Greenwood et al., who also reported competition of [18F]FSPG uptake with both aspartate and glutamate, which are the natural substrates of the EAATs (12,24). Moreover, we observed significant uptake blocking in the presence of l-serine in all 3 cell lines (P = 0.0047 for A549, P = 0.0340 for U-87, and P = 0.0001 for MDA-MB-231 cells, equivalent to 21.7%, 17.0%, and 40.2% [18F]FSPG uptake inhibition, respectively). We also found that the most potent vesicular glutamate transporter inhibitor, rose bengal (25), inhibited the uptake of both [18F]FASu and [18F]FSPG (P value varied but was ≤0.0015 for each comparison) and that it had better blocking efficacy than sulfasalazine in the case of both tracers and across all cell lines studied (Fig. 2; Supplemental Fig. 3). Western blotting indicated expression of EAAT3 and EAAT4 transporters in U-87 and MDA-MB-231 whole-cell lysates (Supplemental Fig. 4). Furthermore, blotting of EAAT1 and EAAT2 indicated bands corresponding to the expression of glycosylated proteins, causing a band shift of 5–15 kDa (26), as well as detection of EAAT1 homodimers and homotrimers in all 3 cell lines tested.
Two-way ANOVA analysis of 1-h uptake of [18F]FASu (A) and [18F]FSPG (B) in A549, U-87, and MDA-MB-231 cells, expressed as percentage of control sample uptake. BCH = 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid. *P < 0.05. **P < 0.01. ***P < 0.001. ****P < 0.0001.
The affinity of [18F]FASu and [18F]FSPG to system  transporter was further studied in a dose-dependent manner in competition assays against the natural substrates of system
transporter was further studied in a dose-dependent manner in competition assays against the natural substrates of system 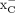 : l-cystine and l-glutamate. Half-maximal inhibitory concentrations of 3.92 ± 0.60 and 3.23 ± 1.01 μM were determined for l-cystine in competition with [18F]FASu and [18F]FSPG, respectively, in A549 cells (Fig. 3; Table 1). These values are less than the values determined in competition with l-glutamate: 10.93 ± 1.00 μM for [18F]FASu and 5.20 ± 1.18 μM for [18F]FSPG. Although the difference in half-maximal inhibitory concentrations for l-cystine was not significant (P = 0.6503), the l-glutamate half-maximal inhibitory concentrations significantly differed (P = 0.0002).
: l-cystine and l-glutamate. Half-maximal inhibitory concentrations of 3.92 ± 0.60 and 3.23 ± 1.01 μM were determined for l-cystine in competition with [18F]FASu and [18F]FSPG, respectively, in A549 cells (Fig. 3; Table 1). These values are less than the values determined in competition with l-glutamate: 10.93 ± 1.00 μM for [18F]FASu and 5.20 ± 1.18 μM for [18F]FSPG. Although the difference in half-maximal inhibitory concentrations for l-cystine was not significant (P = 0.6503), the l-glutamate half-maximal inhibitory concentrations significantly differed (P = 0.0002).
Dose-dependent competition cell uptake assays were performed in A549 (top) and MDA-MB-231 (bottom) cells using 148 kBq per well of either [18F]FASu (A) or [18F]FSPG (B) and increasing concentration of l-cystine or l-glutamate.
Half-Maximal Inhibitory Concentrations (μM) from Tracer Competition Uptake Assays Against l-Cystine and l-Glutamate
In Vivo PET Imaging
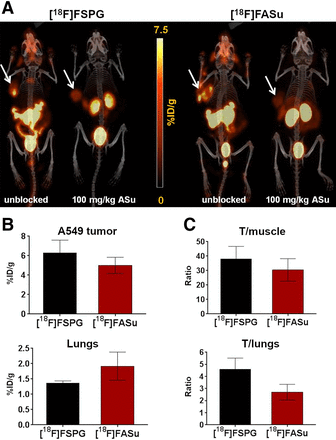
[18F]FASu and [18F]FSPG were evaluated in subcutaneous models of glioblastoma (U-87) and non–small cell lung cancer (A549). The expression of xCT, the light chain subunit of system  , in these tumors was confirmed ex vivo with Western blotting (Supplemental Fig. 5), which revealed a greater abundance of xCT in the A549 tumor lysate. Representative decay-corrected fused PET/CT images and biodistribution data of A549 tumor–bearing Rag2M mice are shown in Figure 4A and Table 2. Both [18F]FASu and [18F]FSPG gave PET images with low background uptake, high image contrast, and clear tumor visualization. Renal clearance was evident from both biodistribution data and images. [18F]FASu and [18F]FSPG both had high pancreatic uptake: 24.93 ± 2.92 and 17.71 ± 3.63 %ID/g, respectively. Both A549 tumor and pancreatic uptake were blocked with coinjection of the nonradioactive standard aminosuberic acid (ASu, 100 mg/kg, intravenously), indicating uptake specificity of the tracers to system
, in these tumors was confirmed ex vivo with Western blotting (Supplemental Fig. 5), which revealed a greater abundance of xCT in the A549 tumor lysate. Representative decay-corrected fused PET/CT images and biodistribution data of A549 tumor–bearing Rag2M mice are shown in Figure 4A and Table 2. Both [18F]FASu and [18F]FSPG gave PET images with low background uptake, high image contrast, and clear tumor visualization. Renal clearance was evident from both biodistribution data and images. [18F]FASu and [18F]FSPG both had high pancreatic uptake: 24.93 ± 2.92 and 17.71 ± 3.63 %ID/g, respectively. Both A549 tumor and pancreatic uptake were blocked with coinjection of the nonradioactive standard aminosuberic acid (ASu, 100 mg/kg, intravenously), indicating uptake specificity of the tracers to system 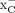 (Table 2) (17). Moreover, ASu coinjection resulted in measurable decreases in tumor-to-muscle, -brain, and -lung uptake ratios for [18F]FASu and a decrease in tumor-to-blood ratio for [18F]FSPG, as indicated in Table 2. Although [18F]FSPG tumor uptake reduction with ASu coinjection was not statistically significant (P = 0.0688), it is worth noting that tumor uptake decreased more than 40% with ASu coinjection. It may also be worth noting that differences in [18F]FSPG tumor-to-brain and -muscle uptake were not significant in the presence of ASu, which is in contrast to the results obtained for [18F]FASu. Tumor uptake of [18F]FASu and [18F]FSPG was 5.00 ± 0.83 and 6.27 ± 1.32 %ID/g, respectively, although it was not significantly different between [18F]FASu and [18F]FSPG (Figs. 4B and 4C). [18F]FSPG had a significantly greater tumor-to-brain ratio than [18F]FASu (P < 0.05); otherwise, there were no significant differences in the biodistribution of these 2 tracers.
(Table 2) (17). Moreover, ASu coinjection resulted in measurable decreases in tumor-to-muscle, -brain, and -lung uptake ratios for [18F]FASu and a decrease in tumor-to-blood ratio for [18F]FSPG, as indicated in Table 2. Although [18F]FSPG tumor uptake reduction with ASu coinjection was not statistically significant (P = 0.0688), it is worth noting that tumor uptake decreased more than 40% with ASu coinjection. It may also be worth noting that differences in [18F]FSPG tumor-to-brain and -muscle uptake were not significant in the presence of ASu, which is in contrast to the results obtained for [18F]FASu. Tumor uptake of [18F]FASu and [18F]FSPG was 5.00 ± 0.83 and 6.27 ± 1.32 %ID/g, respectively, although it was not significantly different between [18F]FASu and [18F]FSPG (Figs. 4B and 4C). [18F]FSPG had a significantly greater tumor-to-brain ratio than [18F]FASu (P < 0.05); otherwise, there were no significant differences in the biodistribution of these 2 tracers.
(A) Uptake of [18F]FSPG and [18F]FASu in A549 tumor–bearing mice at 1 h after injection with and without blocking reagent (100 mg/kg ASu). Arrows indicate location of A549 tumors on fused PET/CT maximum-intensity projection images. (B and C) Two-way ANOVA of tumor-to-muscle and tumor-to-lung ratios (C) and tumor and lung uptake (B) for [18F]FSPG and [18F]FASu in A549 tumor–bearing mice at 1 h after injection. P value varied for each comparison but was always >0.05. T = tumor.
Biodistribution and Tumor-to-Nontarget Ratios of [18F]FASu and [18F]FSPG in A549 Xenograft–Bearing Rag2M Mice
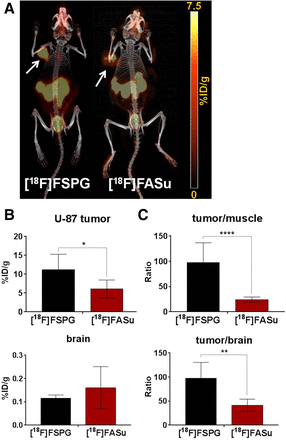
In the case of U-87 tumor–bearing Rag2M mice, the images (Fig. 5A) and biodistribution data (Table 3) resemble those of A549 tumor–bearing animals. Radiofluorinated amino acids [18F]FASu and [18F]FSPG generated good-contrast PET images in xCT-expressing glioblastoma xenografts. The 2 tracers indicated a similar biodistribution pattern in the organs and tissues that were studied (Table 3). In addition to tumor, excretory organs such as the kidneys and bladder showed uptake, indicating a renal excretion pathway (Fig. 5). Blood clearance was rapid, with 0.84 ± 0.50 and 0.59 ± 0.08 %ID/g of [18F]FASu and [18F]FSPG present at 1 h after injection, respectively. [18F]FASu and [18F]FSPG both had high pancreatic uptake, 26.42 ± 11.59 and 16.81 ± 1.38 %ID/g, respectively, because of high xCT expression in this organ (27). U-87 tumor uptake of [18F]FASu and [18F]FSPG at 1 h after injection was 6.05 ± 2.40 and 11.18 ± 4.12 %ID/g, respectively. Glioblastoma xenograft uptake of [18F]FSPG was significantly greater than that of [18F]FASu (P < 0.05), as were tumor-to-muscle and tumor-to-brain uptake ratios (P < 0.0001; Figs. 5B and 5C). Region-of-interest analysis of the dynamic scans with [18F]FASu and [18F]FSPG in U-87 tumor–bearing mice showed a steady increase of tracer uptake by the tumor and a clear delineation from the background activity, as evident on the time–activity curves (Supplemental Fig. 6).
Comparative study. (A) Uptake of [18F]FSPG and [18F]FASu in U-87 tumor–bearing mice at 1 h after injection. Arrows indicate location of U-87 tumors on fused PET/CT maximum-intensity projection images. (B and C) Two-way ANOVA of tumor-to-muscle and tumor-to brain ratios (C) and tumor and brain uptake (B) for [18F]FSPG and [18F]FASu in U-87 tumor–bearing mice at 1 h after injection. *P < 0.05. **P < 0.01. ****P < 0.0001.
Biodistribution and Tumor-to-Nontarget Ratios of [18F]FASu and [18F]FSPG in U-87 Xenograft–Bearing Rag2M Mice
DISCUSSION
Cellular antioxidant machinery functions as a tightly controlled system, in which the purpose is to maintain an intracellular redox balance (28,29). Glutathione is a major endogenous nonenzymatic antioxidant involved in reactive oxygen species neutralization and containment of intracellular OS levels (30). Given the dependency of glutathione biosynthesis on the import of cystine into the cell, system  is considered a marker of cellular OS (10) and, as such, has become an appealing target for therapeutic and imaging purposes. The aim of this study was to directly compare the uptake specificity, biodistribution profile, and imaging utility of 2 tracers targeting system
is considered a marker of cellular OS (10) and, as such, has become an appealing target for therapeutic and imaging purposes. The aim of this study was to directly compare the uptake specificity, biodistribution profile, and imaging utility of 2 tracers targeting system  , both of which have previously been reported as promising noninvasive tools for imaging of intracellular redox status.
, both of which have previously been reported as promising noninvasive tools for imaging of intracellular redox status.
In vitro uptake studies indicate system  –mediated uptake of both [18F]FASu and [18F]FSPG. We found that [18F]FSPG was taken up more readily in vitro and that it had severalfold greater uptake than [18F]FASu in all tested cell lines. We hypothesize that this could be because of reliance of [18F]FSPG on other modes of transport across the plasma membrane, and we tested this hypothesis in a series of specificity assays in which we measured uptake of each tracer in the presence of several different amino acid transporter inhibitors (Fig. 2). In at least 2 of 3 tested cell lines, [18F]FSPG uptake was significantly inhibited by PDC and l-serine (P < 0.05), indicating some involvement of EAATs and numerous serine transporters in the import of this tracer into these cells. Furthermore, we were able to detect the expression of EAAT3 and EAAT4 and predicted glycoproteins of EAAT1 and EAAT2 (26) in these cell lines (Supplemental Fig. 4), further supporting our hypothesis that there might be some reliance on EAATs in the import of [18F]FSPG into these cells. An additional finding in this study was the ability of rose bengal, a known vesicular glutamate transporter inhibitor, to block transport of both [18F]FASu and [18F]FSPG across the plasma membrane in all cell lines studied (P < 0.01; Fig. 2). When coincubated with either one of these 2 radiotracers, rose bengal caused a dose-dependent reduction in intracellular tracer content (Supplemental Fig. 3). Only 10 μM rose bengal was sufficient to prevent greater than 90% of activity from entering A549 cells, which is equivalent to the percentage inhibition achieved by 1 mM sulfasalazine in this study. With these results, the finding that rose bengal inhibits system
–mediated uptake of both [18F]FASu and [18F]FSPG. We found that [18F]FSPG was taken up more readily in vitro and that it had severalfold greater uptake than [18F]FASu in all tested cell lines. We hypothesize that this could be because of reliance of [18F]FSPG on other modes of transport across the plasma membrane, and we tested this hypothesis in a series of specificity assays in which we measured uptake of each tracer in the presence of several different amino acid transporter inhibitors (Fig. 2). In at least 2 of 3 tested cell lines, [18F]FSPG uptake was significantly inhibited by PDC and l-serine (P < 0.05), indicating some involvement of EAATs and numerous serine transporters in the import of this tracer into these cells. Furthermore, we were able to detect the expression of EAAT3 and EAAT4 and predicted glycoproteins of EAAT1 and EAAT2 (26) in these cell lines (Supplemental Fig. 4), further supporting our hypothesis that there might be some reliance on EAATs in the import of [18F]FSPG into these cells. An additional finding in this study was the ability of rose bengal, a known vesicular glutamate transporter inhibitor, to block transport of both [18F]FASu and [18F]FSPG across the plasma membrane in all cell lines studied (P < 0.01; Fig. 2). When coincubated with either one of these 2 radiotracers, rose bengal caused a dose-dependent reduction in intracellular tracer content (Supplemental Fig. 3). Only 10 μM rose bengal was sufficient to prevent greater than 90% of activity from entering A549 cells, which is equivalent to the percentage inhibition achieved by 1 mM sulfasalazine in this study. With these results, the finding that rose bengal inhibits system 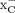 raises the prospect that this compound may induce a therapeutic response (31,32) via glutathione depletion, thus sensitizing the target tissue to other chemotherapeutics (6,33–35).
raises the prospect that this compound may induce a therapeutic response (31,32) via glutathione depletion, thus sensitizing the target tissue to other chemotherapeutics (6,33–35).
[18F]FASu and [18F]FSPG biodistribution and imaging potential was tested in mice bearing U-87 and A549 xenografts. These 2 tumor models were selected for the in vivo experiments because of their disparate uptake pattern in vitro. U-87 cells were on the lower end of the uptake spectra of both tracers, and no increase in blocked uptake of either tracer was evident in vitro. The lung cancer cells, A549, conversely, demonstrated high in vitro uptake of both radiotracers and increasing uptake over time despite oversaturation with sulfasalazine, which is a system 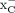 inhibitor.
inhibitor.
In contrast to the in vitro uptake, [18F]FASu and [18F]FSPG have similar biodistribution profiles and excretion pathways, irrespective of the tumor inoculated (A549 or U-87; Tables 2 and 3). Both tracers exhibited low background uptake and moderate to high tumor-to-blood, tumor-to-brain, and tumor-to-muscle ratios. The principal difference between [18F]FASu and [18F]FSPG was found in the greater in vivo tumor uptake of the latter, with significantly greater uptake in the U-87 tumor (Fig. 5B; P < 0.05) yet nonsignificantly greater uptake in A549 tumors (Fig. 4B; P = 0.79), despite greater xCT expression in these tumors (Supplemental Fig. 5). This may be an indication that [18F]FSPG is transported into the cells by the action of alternative transporters, including the EAATs, or system  , in addition to system
, in addition to system  (36). Moreover, an early report on the specificity of [18F]FSPG by Koglin et al. (12) demonstrated that, although [18F]FSPG does predominantly use system
(36). Moreover, an early report on the specificity of [18F]FSPG by Koglin et al. (12) demonstrated that, although [18F]FSPG does predominantly use system 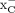 to enter cells, both l-cystine and l-glutamate achieved higher levels of tracer uptake inhibition than a potent system
to enter cells, both l-cystine and l-glutamate achieved higher levels of tracer uptake inhibition than a potent system 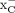 inhibitor, p-carboxy-phenylglycine, indicating that [18F]FSPG may be a suitable substrate for other AATs that include l-glutamate or l-cystine as substrates, such as EAATs (EAAT1, EAAT2, EAAT4, and EAAT5 for l-glutamate and EAAT3 for both) (24). The authors also reported observing a minor competition with either l- or d-aspartate, both of which are substrates along with l-glutamate for the EAAT family members 1–5 (24). Webster et al. (10) performed a similar in vitro uptake inhibition uptake assay with [18F]FASu, and in their study, sulfasalazine was more effective in blocking tracer uptake than l-glutamate, signifying a preference of [18F]FASu for system
inhibitor, p-carboxy-phenylglycine, indicating that [18F]FSPG may be a suitable substrate for other AATs that include l-glutamate or l-cystine as substrates, such as EAATs (EAAT1, EAAT2, EAAT4, and EAAT5 for l-glutamate and EAAT3 for both) (24). The authors also reported observing a minor competition with either l- or d-aspartate, both of which are substrates along with l-glutamate for the EAAT family members 1–5 (24). Webster et al. (10) performed a similar in vitro uptake inhibition uptake assay with [18F]FASu, and in their study, sulfasalazine was more effective in blocking tracer uptake than l-glutamate, signifying a preference of [18F]FASu for system  . The authors reported observing little effect of d-aspartate on [3H]ASu, [3H]Glu, and [3H]Leu uptake, which is in agreement with our finding reported here that [18F]FASu uptake was not affected by the system
. The authors reported observing little effect of d-aspartate on [3H]ASu, [3H]Glu, and [3H]Leu uptake, which is in agreement with our finding reported here that [18F]FASu uptake was not affected by the system  inhibitor PDC.
inhibitor PDC.
Notwithstanding the data presented here, we are aware of other potential explanations for the in vitro uptake fluctuations of [18F]FSPG involving the complex interplay between amino acid transporters and their respective substrates. Inhibition of EAATs has been previously shown to alter intracellular glutamate levels, which are known to affect system 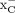 activity (37). Given that fluctuations in intracellular glutamate levels also affect [18F]FSPG retention (38) because of system
activity (37). Given that fluctuations in intracellular glutamate levels also affect [18F]FSPG retention (38) because of system  –mediated exchange, this phenomenon may account for changes in cell retention together with alternative transport mechanisms. Moreover, serine is a precursor for the biosynthesis of cysteine, the levels of which have been shown to affect [18F]FSPG retention in cells through
–mediated exchange, this phenomenon may account for changes in cell retention together with alternative transport mechanisms. Moreover, serine is a precursor for the biosynthesis of cysteine, the levels of which have been shown to affect [18F]FSPG retention in cells through 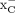 exchange (38).
exchange (38).
Several recent studies have examined the potential use of [18F]FSPG and [18F]FASu for measuring intracellular redox status. McCormick et al. found that in vitro [18F]FSPG uptake in ovarian cancer cells was sensitive to an elevation in glutathione biosynthesis after drug-induced OS (38). Our group recently reported a positive correlation between in vitro [18F]FASu uptake and intracellular glutathione fluctuations in breast cancer models caused by radiation- and diethylmaleate-induced OS (17). Despite the methodic and detailed approach taken in previous studies, a side-by-side study comparing [18F]FASu and [18F]FSPG in the same cancer model became warranted to better understand the subtleties in tracer behavior. The data presented here suggest that the degree of non–system  uptake may vary depending on the cancer model studied and the mode of action of the OS-inducing drug used. Other reports testify to the emerging (poor) prognostic significance of xCT/SLC7A11 overexpression in several different malignancies, including non–small cell lung cancer (39), laryngeal squamous cell carcinoma (40), glioblastoma (41,42), hepatocellular carcinoma (43), colorectal cancer (44,45), and acute myeloid leukemia (46,47). Additional studies speak to the increasing potential of using xCT-targeting therapeutics to sensitize cancers to chemotherapy or radiation therapy (6,33–35,48–50). Therefore, early identification of xCT-expressing tumors would help with patient stratification, treatment plan development, and therapeutic monitoring and intervention because xCT has been recognized as an emerging therapeutic or adjuvant target.
uptake may vary depending on the cancer model studied and the mode of action of the OS-inducing drug used. Other reports testify to the emerging (poor) prognostic significance of xCT/SLC7A11 overexpression in several different malignancies, including non–small cell lung cancer (39), laryngeal squamous cell carcinoma (40), glioblastoma (41,42), hepatocellular carcinoma (43), colorectal cancer (44,45), and acute myeloid leukemia (46,47). Additional studies speak to the increasing potential of using xCT-targeting therapeutics to sensitize cancers to chemotherapy or radiation therapy (6,33–35,48–50). Therefore, early identification of xCT-expressing tumors would help with patient stratification, treatment plan development, and therapeutic monitoring and intervention because xCT has been recognized as an emerging therapeutic or adjuvant target.
The main objective of this work was to compare the 2 tracers with respect to their specificity for the target transporter and tumor visualization potential. [18F]FSPG is currently in use in pilot clinical studies (51–53) and is showing promise as a marker of therapeutic response in the preclinical setting in high-grade serous ovarian cancer models (54). [18F]FASu performed as well as [18F]FSPG as an in vivo oncologic imaging agent in a preclinical setting and may warrant further consideration for clinical use.
CONCLUSION
We found that [18F]FSPG is taken up more readily in vitro by all cell lines tested, whereas ex vivo biodistribution was generally equivalent between [18F]FSPG and [18F]FASu, with the exception of greater [18F]FSPG uptake in glioblastoma xenografts. This study examined the participation of alternative AATs, such as system  , in the transport of both tracers. Nevertheless, we found that [18F]FSPG and [18F]FASu have comparable biodistribution and tumor-imaging ability in vivo, supporting the further pursuit of either tracer for non-[18F]FDG oncologic imaging.
, in the transport of both tracers. Nevertheless, we found that [18F]FSPG and [18F]FASu have comparable biodistribution and tumor-imaging ability in vivo, supporting the further pursuit of either tracer for non-[18F]FDG oncologic imaging.
DISCLOSURE
This work was supported by the CIHR (grants 201403COP and 329895). TRIUMF receives federal funding via a contribution agreement with the National Research Council of Canada. This work was supported in part by the BC Leading Edge Endowment Fund. Milena Colovic received support from an NSERC CREATE IsoSiM fellowship, grant 448110, competition year 2014. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: How do the 2 system  imaging agents, [18F]FASu and [18F]FSPG, compare with respect to their uptake specificity and tumor imaging potential?
imaging agents, [18F]FASu and [18F]FSPG, compare with respect to their uptake specificity and tumor imaging potential?
PERTINENT FINDINGS: Our in vitro specificity assays indicated greater overall uptake of [18F]FSPG than of [18F]FASu but also potential participation of other AA transporters. Moreover, we found that rose bengal inhibited uptake of both tracers in vitro. In preclinical subjects, however, both tracers were successful at visualizing glioma (U-87) and lung cancer (A549) xenografts and had comparable tumor uptake and pharmacokinetics.
IMPLICATIONS FOR PATIENT CARE: [18F]FASu and [18F]FSPG are redox PET tracers that, when translated into the clinic, have the potential to serve as indicators of the redox state of the lesion, companion diagnostics, and, potentially, patient prognosis.
ACKNOWLEDGMENTS
We thank the TRIUMF TR13 cyclotron team, in particular David Prevost, Linda Graham, and Samuel Varah, for technical assistance.
Footnotes
Published online Apr. 28, 2023.
- © 2023 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication December 5, 2022.
- Revision received March 21, 2023.