Visual Abstract
Abstract
We investigated the effects of blood glucose levels on the performance of 18F-FDG PET/CT for detecting an infection focus in patients with bacteremia. Methods: A total of 322 consecutive patients with bacteremia who underwent 18F-FDG PET/CT between 2010 and 2021 were included. Logistic regression analysis was performed to evaluate the association between finding a true-positive infection focus on 18F-FDG PET/CT and blood glucose level, type of diabetes, and use of hypoglycemic medication. C-reactive protein, leukocyte count, duration of antibiotic treatment, and type of isolated bacteria were considered as well. Results: Blood glucose level (odds ratio, 0.76 per unit increase; P = <0.001) was significantly and independently associated with 18F-FDG PET/CT outcome. In patients with a blood glucose level between 3.0 and 7.9 mmol/L (54–142 mg/dL), the true-positive detection rate of 18F-FDG PET/CT varied between 61% and 65%, whereas in patients with a blood glucose level between 8.0 and 10.9 mmol/L (144–196 mg/dL), the true-positive detection rate decreased to 30%–38%. In patients with a blood glucose level greater than 11.0 mmol/L (200 mg/dL), the true-positive detection rate was 17%. In addition to C-reactive protein (odds ratio, 1.004 per point increase; P = 0.009), no other variables were independently associated with 18F-FDG PET/CT outcome. Conclusion: In patients with moderate to severe hyperglycemia, 18F-FDG PET/CT was much less likely to identify the focus of infection than in normoglycemic patients. Although current guidelines recommend postponing 18F-FDG PET/CT only in cases of severe hyperglycemia with glucose levels greater than 11 mmol/L (200 mg/dL), a lower blood glucose threshold seems to be more appropriate in patients with bacteremia of unknown origin and other infectious diseases.
Bacteremia is defined by the presence of viable bacteria in the bloodstream. With an incidence between 100 and 200 cases per 100,000 people per year, bacteremia is one of the most common causes of hospital admission (1,2). As source control is the most important treatment for bacteremia, the mortality rate of bacteremia strongly depends on the ability to locate the source of infection (3). When no source can be identified, patients are diagnosed with bacteremia of unknown origin (4).
18F-FDG PET/CT has proven to be very useful in diagnosing numerous infectious diseases, including infection foci in patients with bacteremia of unknown origin (5,6). As leukocytes and other inflammatory cells such as cytokines are recruited to infection sites and usually consume more glucose than does the surrounding tissue, infection foci are often readily visible on 18F-FDG PET/CT (7). Most bacteria consume glucose as well (8).
Even though glucose metabolism plays a vital role in 18F-FDG PET/CT, the effect of hyperglycemia on the diagnostic performance of 18F-FDG PET/CT in patients with infectious disease remains poorly understood. Theoretically, hyperglycemia can cause reduced cellular 18F-FDG uptake due to direct competition with plasmatic glucose at glucose binding sites (9). Hyperglycemia can also lead to hyperinsulinemia, which causes upregulation of glucose type 4 transporters and subsequently higher skeletal and myocardial 18F-FDG uptake (10). Both mechanisms could potentially cause false-negative results in hyperglycemic patients with infectious disease, but literature on the clinical consequences of hyperglycemia on the diagnostic accuracy of 18F-FDG PET/CT is limited and conflicting (11–17). Infection foci may also show a large variation in location and in 18F-FDG avidity. For example, endocarditis may be obscured by physiologic high myocardial uptake due to hyperglycemia and osteomyelitis by just a marginally elevated 18F-FDG uptake compared with the background in low-grade infections. Additionally, the prevalence of diabetes mellitus in the community is rapidly increasing, especially in high-income countries (18).
In the current PET imaging guidelines, the recommended upper threshold of plasma glucose before clinical 18F-FDG PET/CT studies are performed is 11 mmol/L (200 mg/dL) (15–18). For research studies, an upper glucose threshold of 8.3 mmol/L (150 mg/dL) is recommended (19). Although these thresholds are widely applied, the effects of moderate (7.8–10.0 mmol/L or 140–180 mg/dL) to severe hyperglycemia (>10.0 mmol/L or >180 mg/dL) on the diagnostic performance of 18F-FDG PET/CT remain unclear, especially in patients with infectious disease (20).
Therefore, the aim of this study was to assess the effects of hyperglycemia on the diagnostic performance of 18F-FDG PET/CT in patients with bacteremia of unknown origin.
MATERIALS AND METHODS
Study Design and Patients
The electronic patient information system of University Medical Center Groningen was searched for all patients who underwent 18F-FDG PET/CT between 2010 and 2021 to find the focus of infection using the keywords “sepsis,” “bacteremia,” “infection,” “fever,” and “blood culture.” All patients for whom bacteremia was confirmed by blood cultures taken within 2 mo before 18F-FDG PET/CT were included. Patients with negative blood cultures or blood cultures that were considered contaminated by medical microbiologists were excluded. Follow-up 18F-FDG PET/CT scans and 18F-FDG PET/CT scans performed for other reasons than locating the source of infection, such as oncologic follow-up, were excluded as well. The local institutional review board approved this retrospective, single-center study and waived the requirement for written informed consent (Institutional Review Board number 201700145).
Patient Data Review and Reference Standard
The medical files of all patients were reviewed for relevant clinical and biochemical data (age, sex, medical history [including the presence of diabetes], laboratory values [blood glucose before 18F-FDG PET/CT, C-reactive protein (CRP), leukocyte count, type of isolated bacteria]; duration of hospital stay; use of hypoglycemic medication, antibiotics, and immunosuppressive medication; final diagnosis at hospital discharge; and 6-mo follow-up data).
18F-FDG PET/CT Acquisition
All scans were performed using an integrated PET/CT system (Biograph mCT 40- or 64-slice PET/CT or Biograph Vision PET/CT; Siemens) with 3 min per bed position. Low-dose CT was performed for attenuation correction and anatomic mapping at 100 kV and 30 mAs. Data acquisition and reconstruction were in accordance with European Association of Nuclear Medicine/Research 4 Life guidelines (19). In 60 patients, concomitant full-dose CT of the neck, thorax, or abdomen was performed with a constant tube potential of 100 or 120 kV and automatic adjustment of milliampere seconds in the z-direction.
Patients fasted for a minimum of 6 h, and blood glucose concentration was ensured to be less than 11 mmol/L (200 mg/dL) before 3 MBq of 18F-FDG/kg of body weight were administered intravenously. In 7 patients, 18F-FDG PET/CT was performed even though the blood glucose level was greater than 11 mmol/L because of clinical urgency and poorly manageable diabetes. When there was a clinical suspicion of infective endocarditis, patients were also prepared with a high-fat, low-carbohydrate diet for at least 24 h. In some patients suspected of endocarditis, a heparin loading dose of 50 IU/kg was administered 15 min before imaging to reduce myocardial 18F-FDG uptake. PET/CT imaging was performed approximately 60 min after intravenous 18F-FDG administration.
18F-FDG PET Interpretation and Reference Standard
18F-FDG PET/CT scans were interpreted by experienced nuclear medicine physicians as part of routine clinical care using Syngo.Via software (Siemens Healthcare). All 18F-FDG PET/CT scans were reevaluated by one of the authors. In the case of any doubt or inconsistencies with previous reports and 18F-FDG PET/CT images, the images were reevaluated by another author, a nuclear medicine physician, who was unaware of the original 18F-FDG PET/CT interpretations and the results of all other imaging and clinical, laboratory, and microbiologic tests. 18F-FDG PET/CT scans showing at least 1 18F-FDG–avid lesion localized to an area that did not correspond to physiologic biodistribution of 18F-FDG and did not suggest a pathology other than infection were considered to be positive for an infection focus. The final clinical diagnosis at hospital discharge was used as a reference standard for 18F-FDG PET/CT results. This clinical diagnosis was based on all clinically available data including histology or microbiology reports; other imaging results, such as ultrasonography or MRI, confirming the infection focus found on 18F-FDG PET/CT; and clinical follow-up and treatment outcome for at least 6 mo. The final diagnosis was never based on 18F-FDG PET/CT results alone.
Statistical Analyses
Continuous variables were checked for normal distribution using Shapiro–Wilk tests. Data were presented as mean ± SD or median with interquartile range (IQR) for normally or nonnormally distributed data, respectively. The sensitivity, specificity, positive predictive value, and negative predictive value of 18F-FDG PET/CT for detecting an infection focus were calculated, along with 95% CI. Age, sex, medical history (including the presence of diabetes), laboratory values (blood glucose before 18F-FDG PET/CT, CRP, leukocyte count, type of isolated bacteria), and duration of hospital stay, as well as the use of hypoglycemic medication, antibiotics, and immunosuppressive medication, were analyzed with univariable logistic regression as independent variables and the 18F-FDG PET/CT result as the dependent variable. The 18F-FDG PET/CT result based on the final discharge diagnosis was either true-positive or not true-positive. As such, all true-positive results were analyzed against true-negative, false-positive, and false-negative results. Corresponding odds ratios (ORs) and 95% CIs were calculated, and P values of less than 0.05 were considered to be statistically detectable. Variables with a P value of 0.10 or less on univariable analysis were included in the backward multivariable logistic regression model. All statistical analyses were performed using IBM Statistical Package for the Social Sciences, version 28.
RESULTS
Patient Population
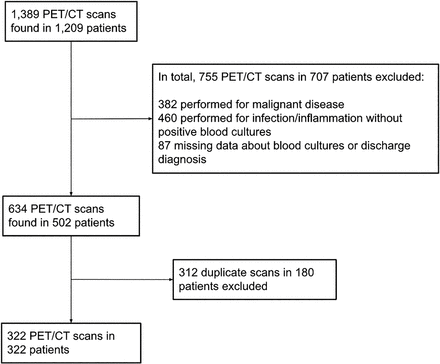
In total, 1,389 18F-FDG PET/CT scans from 1,209 individual patients were potentially eligible for inclusion. After the inclusion and exclusion criteria were reviewed, 322 18F-FDG PET/CT scans from 322 patients were finally included (Fig. 1). These included 206 men and 116 women, with a median age of 63.5 y (IQR, 20 y) (Table 1). Of these, 66 patients had type 2 diabetes (20%), 7 patients had type 1 diabetes (2%), and 4 patients had new-onset diabetes after transplantation (1%). Fifty-four patients (17%) were using insulin, 27 patients (8%) metformin, and 6 patients (2%) sulfonylurea derivatives. Ninety-nine patients (31%) were using immunosuppressive medication such as prednisolone or tacrolimus. The median duration of antibiotic treatment before 18F-FDG PET/CT was 7 d (IQR, 7 d); the median duration between the last positive blood cultures and 18F-FDG PET/CT was 6 d (IQR, 7 d), and the median duration of the hospital stay was 23 d (IQR, 25 d). The median CRP level was 87 mg/L (IQR, 116 mg/L), with a median leukocyte count of 8.9 × 109/L (IQR, 6.7 × 109/L) and a median blood glucose level of 5.4 (IQR, 1.8 mmol/L). Most patients had bacteremia caused by Staphylococcus aureus (30%), followed by gram-negative rods (20%) and Streptococci (14%). The in-hospital mortality rate was 14%.
Patient inclusion tree.
Patient Characteristics
Diagnostic Performance of 18F-FDG PET/CT
According to the reference standard, 18F-FDG PET/CT yielded 188 true-positive results, 19 false-positive results, 78 true-negative results, and 37 false-negative results for finding the infection focus. The resulting sensitivity was 83.6% and specificity was 80.4%, with a positive predictive value of 90.8% and a negative predictive value of 67.8% (Table 2). The most commonly diagnosed (true-positive) infections were spondylodiskitis or sacroiliitis (38 patients, 12%); other musculoskeletal infections, such as septic arthritis or osteomyelitis (22 patients, 7%); and pulmonary infections (21 patients, 7%) (Table 3). The most common false-positive diagnoses were infected hematomas shortly after surgery (n = 3, 1%), for which elevated 18F-FDG uptake caused by inflammation was misinterpreted as an infection; musculoskeletal infection such as mediastinitis shortly after sternotomy (n = 3, 1%), for which 18F-FDG avidity caused by postoperative changes was misinterpreted as an infection; and pulmonary infection (n = 3), for which 18F-FDG PET/CT mostly showed mildly elevated 18F-FDG uptake in pleural effusion possibly caused by infection, but the final diagnosis was bacteremia of unknown origin. The most common false-negative diagnoses included endocarditis (16 patients, 5%) and infected venous access ports (4 patients, 1%) (21).
Diagnostic Performance of 18F-FDG PET/CT for Detecting Infection Focus
True-Positive, False-Positive, True-Negative, and False-Negative Infections Based on 18F-FDG PET/CT Results and Final Discharge Diagnosis
Factors Associated with 18F-FDG PET/CT Outcome
On univariable logistic regression, not having diabetes (OR, 1.67; P = 0.050), an increase in CRP level (OR, 1.003 per unit increase; P = 0.003), an increase in blood glucose level (OR, 0.82 per unit increase; P = 0.003), use of immunosuppressants (OR, 0.61; P = 0.044), and bacteremia caused by Enterococci (OR, 0.028; P = 0.028) were significantly associated with detecting a true-positive infection focus on 18F-FDG PET/CT (Table 4). On multivariable logistic regression, only an increase in CRP level (OR, 1.004 per unit increase; P = 0.009) and blood glucose level (OR, 0.76 per unit increase; P = <0.001) before 18F-FDG PET/CT remained independently associated with detecting an infection focus on 18F-FDG PET/CT.
Factors Associated with Detecting True-Positive Infection Focus on 18F-FDG PET/CT
In 253 patients with a blood glucose level between 3.0 and 7.9 mmol/L (54–142 mg/dL), the true-positive detection rate of 18F-FDG PET/CT varied between 61% and 65% (Fig. 2). In 57 patients with a blood glucose level between 8.0 and 10.9 mmol/L (144–196 mg/dL), the true-positive detection rate decreased to 30%–38%. 18F-FDG PET/CT was performed on 7 patients with a blood glucose level greater than the recommended threshold of 11.0 mmol/L (200 mg/dL). In only 1 of these patients (17%) was a true-positive infection focus found. Two patient examples are shown in Figures 3 and 4.
Blood glucose level and detection rate of true-positive infection focus on 18F-FDG PET/CT.
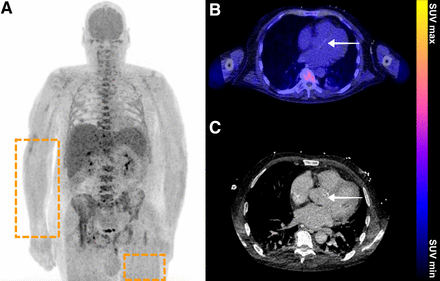
A 64-y-old man presented to hospital with septic shock. His medical history showed type 2 diabetes mellitus, hypertension, and myocardial infarction. At presentation, his CRP level was 78 mg/L, and his leukocyte count was 13.3 × 109/L. Blood cultures were positive for S. aureus. To identify focus of infection, 18F-FDG PET/CT was performed. Before 18F-FDG PET/CT, his blood glucose level measured 11.1 mmol/L (200 mg/dL). (A) Coronal maximum-intensity-projection 18F-FDG PET showed diffusely increased 18F-FDG uptake in skeletal muscle (rectangles), which made images difficult to interpret. High glucose level was deemed to be most probable cause for this elevated skeletal muscle uptake. (B and C) Axial 18F-FDG PET/CT (B) and low-dose CT (C) did not show elevated 18F-FDG uptake at aortic valve (arrows), even though valvular vegetation of aortic valve was seen on transesophageal ultrasound, suggestive of endocarditis. Thus, 18F-FDG PET/CT result was deemed to be false-negative. Despite antibiotic treatment, patient died several days after 18F-FDG PET/CT was performed. Autopsy was not performed.
A 64-y-old woman presented to hospital with painful left wrist and fever. Her medical history showed bilateral hip replacement and internal fixation of left wrist fracture with plates and screws earlier that year. On admittance, she had CRP of 274 mg/L and leukocyte count of 18.4 × 109/L. Blood cultures were positive for S. aureus. 18F-FDG PET/CT was performed to identify primary infection focus and potential metastatic foci. By accident, insulin was administered to patient shortly before 18F-FDG PET/CT was performed. (A) Coronal maximum-intensity projection showed diffusely increased 18F-FDG uptake in skeletal muscle (rectangle), rendering 18F-FDG PET images difficult to interpret. Myocardium also showed elevated 18F-FDG uptake (white arrow). Pathologic 18F-FDG uptake was seen at left wrist (yellow arrow). (B and C) On radiography (B), wrist uptake on PET was interpreted as infected osteosynthetic material (arrow), also visible on axial 18F-FDG PET/CT (C, arrow). Plates and screws of left wrist were surgically removed and cultured. These cultures were also positive for S. aureus. Despite antibiotic treatment, patient died of septic shock 1 wk after 18F-FDG PET/CT was performed.
Effect of Hypoglycemic Medication Use on 18F-FDG PET/CT Outcome
On univariable logistic regression, only the use of metformin approached statistical significance (OR, 0.48; P = 0.077). Use of insulin (OR, 0.71; P = 0.26) or sulfonylurea derivatives (OR, 2.11; P = 0.52) was not significantly associated with 18F-FDG PET/CT outcome. The individual dosages were not specifically analyzed.
DISCUSSION
The results of this study show that managing blood glucose before performing 18F-FDG PET/CT has a large impact on its ability to identify the infection focus in patients with bacteremia of unknown origin.
On univariable and multivariable logistic regression, a higher blood glucose level was associated with significantly lower odds of detecting an infection focus on 18F-FDG PET/CT (OR, 0.76; P = 0.003). The detection rate of 18F-FDG PET/CT was 60%–65% in patients with a blood glucose level less than 8.0 mmol/L (144 mg/dL) but decreased to 14%–38% in patients with a blood glucose level less than 8.0 mmol/L.
Most nuclear imaging guidelines recommend that 18F-FDG PET/CT not be postponed unless the glucose level exceeds an upper threshold of 11 mmol/L (200 mg/dL) (22). However, this threshold also depends on the indication for 18F-FDG PET/CT and the setting in which 18F-FDG PET/CT is performed. For example, the recommended upper threshold for brain imaging is 8.8 mmol/L (160 mg/dL), and the European Association of Nuclear Medicine recommends an upper threshold of 7.0–8.3 mmol/L (126–150 mg/dL) for clinical trials (16). The procedure guideline for tumor imaging from the Society of Nuclear Medicine and Molecular Imaging recommends an upper threshold between 8.3 and 11 mmol/L (150–200 mg/dL) (23). The rationale behind these differences in upper threshold is unclear. Also, there is no distinction between the glucose threshold in patients who undergo 18F-FDG PET/CT for oncologic disease and that in patients who undergo 18F-FDG PET/CT for infectious disease, even though the physiology behind elevated 18F-FDG uptake in oncologic and infectious lesions is different. Cancer cells consume much glucose because of increased proliferation and inefficient metabolism with glycolysis instead of oxidative phosphorylation as their main metabolic pathway (24). An infection focus is thought to be 18F-FDG–avid mainly because of the recruitment of other metabolically active cells such as granulocytes. Additionally, most bacteria consume glucose and therefore 18F-FDG. As the 18F-FDG avidity of different types of bacteria also differs, it could be hypothesized that 18F-FDG uptake in infection foci with more fulminant bacteria, such as S. aureus, is less easily affected by high blood glucose levels than are the less fulminant pathogens (8). On univariable regression, an Enterococcus bacteremia was also significantly associated with lower odds of identifying an infection focus on 18F-FDG PET/CT (OR, 0.45; P = 0.028). However, the statistical significance of this variable was not maintained in the multivariate model.
In both cancer and infectious disease, high blood glucose can cause reduced cellular 18F-FDG uptake due to direct competition with plasmatic glucose at glucose binding sites and upregulation of the glucose transporters of other tissues, resulting in a lower lesion-to-background ratio (9). Some studies also suggest that cancer cells are less easily saturated by glucose than are other tissues (14,25).
Not having diabetes was significantly associated with identifying an infection focus on 18F-FDG PET/CT with univariable logistic regression (OR, 1.67; P = 0.050) but not with multivariable logistic regression. This finding suggests that diabetic patients may not be at a disadvantage for detection of their infection focus on 18F-FDG PET/CT when their blood glucose is properly managed. This includes fasting for 4–6 h and also making sure that no external glucose is intravenously administered. Rapid-acting insulin should be administered only up to 4 h before 18F-FDG PET/CT, short-acting insulin should be administered within 6 h, and intermediate- or long-acting insulin should not be used on the day 18F-FDG PET/CT is scheduled (26). Interestingly, the use of hypoglycemic medication such as metformin or insulin was not statistically associated with 18F-FDG PET/CT outcome, implying that a satisfactory effect of these medications on glucose level and not the use of these medications alone is important for 18F-FDG PET/CT outcome.
In addition to blood glucose, CRP was the only other factor independently associated with 18F-FDG PET/CT outcome. This finding is in line with previous studies, which also found that a higher CRP increases the chance of identifying an infection focus on 18F-FDG PET/CT in patients with bacteremia (27–29). Although a previous study of our own (30) showed a statistically detectable relation between duration of antibiotic use and 18F-FDG PET/CT outcome, this relation was not shown in our current study (OR, 0.99 per day; P = 0.25), perhaps because much more recent patients were included in the current study. Our hospital became more selective in only providing specialized tertiary care over the past few years, resulting in the inclusion of more patients with comorbidities such as infected vascular grafts or prostheses requiring chronic antibiotic suppressive therapy or immunocompromised patients requiring prophylactic antibiotic treatment.
Previous literature on the effects of hyperglycemia on 18F-FDG PET/CT outcome in patients with infectious disease is limited. In a study by Rabkin et al., 123 patients with infection or inflammation were included (12). Nineteen patients had a glucose level greater than 10 mmol/L (180 mg/dL), and in 11 patients (58%), a true-positive infection or inflammation focus was found. No statistically detectable difference was found in the number of false-negative results between normoglycemic and hyperglycemic patients. Because patients with both inflammatory and infectious diseases were included, it is unclear how many of these 11 patients had infectious disease. Additionally, not all patients had an infection of unknown origin. Twenty-six of 123 patients (21%) underwent 18F-FDG PET/CT to evaluate their diabetic foot. A higher number of true-positive results in hyperglycemic patients than in our patient population could have resulted from the fact that hyperglycemia is more likely in patients with a diabetic foot than in general patients with bacteremia of unknown origin, and the a priori chance of detecting an infection in patients with a chronic wound such as diabetic foot is likely higher than in all patients with bacteremia of unknown origin (12).
In a large metaanalysis by Eskian et al., which was conducted to evaluate the effect of prescan blood glucose levels on the SUVs of healthy tissue, 8,380 patients were included with SUV measurements of the liver, brain, muscle, blood pool, or tumors (14). Patients were categorized in 5 groups based on blood glucose: less than 109 mg/dL (euglycemic), 110–125 mg/dL (mild hyperglycemia), 126–150 mg/dL (moderate hyperglycemia), 151–200 mg/dL (high moderate hyperglycemia), and greater than 200 mg/dL (severe hyperglycemia). Eskian et al. concluded that blood glucose affected 18F-FDG uptake in tumors only when the glucose level was greater than 11 mmol/L (200 mg/dL). Blood glucose showed a statistically detectable negative correlation with 18F-FDG uptake in the muscles and brains in all hyperglycemic groups and a statistically detectable positive correlation in all hyperglycemic patients with 18F-FDG uptake in the blood pool and livers. Unfortunately, patients with infectious disease were not included, and the underlying mechanism of 18F-FDG avidity for infectious and oncologic lesions is different.
Our study was compromised by some limitations. First, because of the retrospective nature of this study, selection bias may have occurred. Although all patients were selected using objective criteria, not all patients from our hospital with bacteremia underwent 18F-FDG PET/CT because this indication was set by the treating physician. Second, the reference standard for 18F-FDG PET/CT, namely the clinical diagnosis at hospital discharge, was suboptimal because this diagnosis also included 18F-FDG PET/CT results. However, this diagnosis was never based on 18F-FDG PET/CT results alone. For future research, it would be interesting to conduct quantitative measurements of infection foci in patients with bacteremia to examine whether there may be not only a lower detection rate on 18F-FDG PET/CT but also a lower 18F-FDG uptake in infection foci in patients with moderate to hyperglycemia than in normoglycemic patients. The difference in the 18F-FDG avidity of infections caused by different types of bacteria (e.g., Enterococci vs. S. aureus) could be investigated as well.
KEY POINTS
QUESTION: Does moderate hyperglycemia affect the ability of 18F-FDG PET/CT to find the infection focus in patients with bacteremia of unknown origin?
PERTINENT FINDINGS: In our study including 322 patients, the true-positive detection rate of 18F-FDG PET/CT was 61%–65% in patients with a blood glucose level of 3.0–7.9 mmol/L (54–142 mg/dL), 30%–38% for a level of 8.0–10.9 mmol/L (144–196 mg/dL), and 17% for a level greater than 11.0 mmol/L (200 mg/dL).
IMPLICATIONS FOR PATIENT CARE: Nuclear medicine physicians should be aware that even only moderate hyperglycemia negatively affects the ability of 18F-FDG PET/CT to locate the source of infection in patients with bacteremia of unknown origin. This may warrant adjustment of current scanning protocols, in which an upper prescan blood glucose threshold of 11.0 mmol/L (200 mg/dL) is generally maintained.
CONCLUSION
In patients with bacteremia with a glucose level greater than 8 mmol/L (144 mg/dL), 18F-FDG PET/CT was much less likely to identify the infection focus. Although current guidelines recommend postponing 18F-FDG PET/CT only in cases of severe hyperglycemia with a glucose level greater than 11 mmol/L (200 mg/dL), the diagnostic power of 18F-FDG PET/CT already seems to be affected in patients with only moderate hyperglycemia (>8.0 mmol/L or 144 mg/dL). Stricter blood glucose management seems appropriate in patients who undergo 18F-FDG PET/CT for bacteremia of unknown origin or other infectious diseases. Diabetic patients with adequately controlled blood glucose levels had detection rates on 18F-FDG PET/CT similar to those of nondiabetic patients.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
Footnotes
Published online Jun. 29, 2023.
- © 2023 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication October 1, 2022.
- Revision received April 14, 2023.