Visual Abstract
Abstract
The integrin αvβ6, an epithelium-specific cell surface receptor, is overexpressed on numerous malignancies, including the highly lethal pancreatic ductal adenocarcinomas. Here, we developed and tested a novel αvβ6-targeting peptide, DOTA-5G (1) radiolabeled with 68Ga, for PET/CT imaging and 177Lu for treatment. With the goal to develop a radiotheranostic, further modifications were made for increased circulation time, renal recycling, and tumor uptake, yielding DOTA-albumin-binding moiety-5G (2). Methods: Peptides 1 and 2 were synthesized on solid phase, and their affinity for αvβ6 was assessed by enzyme-linked immunosorbent assay. The peptides were radiolabeled with 68Ga and 177Lu. In vitro cell binding, internalization, and efflux of 68Ga-1 and 177Lu-2 were evaluated in αvβ6-positive BxPC-3 human pancreatic cancer cells. PET/CT imaging of 68Ga-1 and 68Ga-2 was performed on female nu/nu mice bearing subcutaneous BxPC-3 tumors. Biodistribution was performed for 68Ga-1 (1 and 2 h after injection), 68Ga-2 (2 and 4 h after injection), and 177Lu-1 and 177Lu-2 (1, 24, 48, and 72 h after injection). The 177Lu-2 biodistribution data were extrapolated for human dosimetry data estimates using OLINDA/EXM 1.1. Therapeutic efficacy of 177Lu-2 was evaluated in mice bearing BxPC-3 tumors. Results: Peptides 1 and 2 demonstrated high affinity (<55 nM) for αvβ6 by enzyme-linked immunosorbent assay. 68Ga-1, 68Ga-2, 177Lu-1, and 177Lu-2 were synthesized in high radiochemical purity. Rapid in vitro binding and internalization of 68Ga-1 and 177Lu-2 were observed in BxPC-3 cells. PET/CT imaging and biodistribution studies demonstrated uptake in BxPC-3 tumors. Introduction of the albumin-binding moiety in 177Lu-2 resulted in a 5-fold increase in tumor uptake and retention over time. Based on the extended dosimetry data, the dose-limiting organ for 177Lu-2 is the kidney. Treatment with 177Lu-2 prolonged median survival by 1.5- to 2-fold versus controls. Conclusion: 68Ga-1 and 177Lu-2 demonstrated high affinity for the integrin αvβ6 both in vitro and in vivo, were rapidly internalized into BxPC-3 cells, and were stable in mouse and human serum. Both radiotracers showed favorable pharmacokinetics in preclinical studies, with predominantly renal excretion and good tumor–to–normal-tissue ratios. Favorable human dosimetry data suggest the potential of 177Lu-2 as a treatment for pancreatic ductal adenocarcinoma.
Despite exhaustive testing and some encouraging advances in first- and second-line treatments, pancreatic ductal adenocarcinoma (PDAC) remains the fourth leading cause of cancer-related deaths, with a 5-y survival below 10% (1,2). This dire reality is also in part due to diagnosis at an advanced stage of the disease and the poor reliability of current standard imaging approaches. Therefore, there clearly remains an urgent unmet clinical need for more effective molecularly targeted diagnostics and therapeutics.
The heterodimeric transmembrane receptor integrin αvβ6 has been identified as a potential molecular target; it is an epithelium-specific cell surface receptor that is undetectable in healthy adult epithelium but is significantly upregulated in a wide range of epithelium-derived cancers, including PDAC (3–10). In fact, αvβ6 was initially identified in PDAC with nearly uniform high expression among patient samples screened; moreover, metastatic lesions demonstrate further highly upregulated expression of αvβ6 when compared with the primary tumor, and αvβ6 is undetectable in normal pancreas (11). These traits further underscore the potential of αvβ6 as an attractive target for both early detection and targeted delivery of a therapeutic payload in PDAC.
Radiotheranostics combines molecular imaging with targeted radionuclide therapy, often using the same targeting ligand, and has shown efficacy in several cancers (12,13). Over the last few years, we have seen an exponential growth in the development and acceptance of radiotheranostics for applications in oncology. For example, 68Ga-DOTATATE for imaging of neuroendocrine tumors and 177Lu-DOTATATE for peptide receptor radionuclide therapy were the first radiotheranostic peptides to be approved by the Food and Drug Administration, in 2018 (14). More recently, 18F-DCFPyL and 68Ga-PSMA-11 gained approval for imaging, as did 177Lu-PSMA-617 for treatment of prostate-specific membrane antigen–positive metastatic castration-resistant prostate cancer (15).
Several groups, including our own, have developed molecular imaging agents to target the integrin αvβ6, and several promising agents have advanced to clinical trials for imaging cancer and fibrosis (16–20). Building on over a decade of work by the Sutcliffe laboratory to develop αvβ6-targeted molecular imaging agents (17,21,22), we now propose to address the clear unmet need for new therapies for PDAC using a radiotheranostic strategy. We present a novel molecularly targeted radiotheranostic approach via the integrin αvβ6 for peptide receptor radionuclide therapy. DOTA-5G (1) was designed to selectively target the integrin αvβ6; in addition, with the goal to increase blood residence time, tumor uptake, and renal recycling, an albumin-binding moiety (ABM) was incorporated in the peptide to yield DOTA-ABM-5G (2) (Fig. 1). The peptides were synthesized on solid phase, labeled with 68Ga (half-life, 68 min; Eβ+ [max], 900 keV [23%]) for imaging and 177Lu (half-life, 6.7 d; Eβ− [max], 490 keV; Eγ, 208 keV [11%]) for therapy, and evaluated in vitro (for cell binding, internalization, and efflux) in αvβ6-expressing BxPC-3 human pancreatic cancer cells. Albumin binding and stability in mouse and human serum were determined. In vivo PET/CT imaging and biodistribution studies were performed on mice bearing BxPC-3 tumors xenografts, and αvβ6-specific targeting was confirmed by blocking studies. Human dosimetry was estimated from the extended biodistribution data, and therapeutic efficacy was evaluated in mice bearing BxPC-3 tumors.
Chemical structures of 1 and 2. The peptide is indicated in red, DOTA chelator in green, and ABM in blue.
MATERIALS AND METHODS
Chemistry and Radiochemistry
Peptide synthesis on solid phase (23) and radiolabeling and formulation of 68Ga- and 177Lu-labeled peptides are described in Supplemental Section 2 (supplemental materials are available at http://jnm.snmjournals.org).
In Vitro Experiments
Enzyme-linked immunosorbent assays (ELISA) (23,24) and cell binding and internalization, albumin binding (23), and serum stability (25) tests followed previously published procedures as described in Supplemental Section 3.
In Vivo Imaging, Biodistribution, and Targeted Radionuclide Therapy
All animal studies were performed according to procedures approved by the University of California Davis Institutional Animal Care and Use Committee. BxPC-3 cells (5 × 106) were implanted subcutaneously into the left shoulder of 6- to 8-wk-old female nu/nu nude mice (Charles River Laboratories) and allowed to grow for 3 wk (imaging and biodistribution) or approximately 2 wk (therapy).
For imaging, the radiotracer (68Ga-1 or 68Ga-2, 7.4–9.25 MBq) in phosphate-buffered saline (PBS) solution (150 μL, pH 7.2) was injected into the tail vein of mice (n = 3/time point/radiotracer) anesthetized with 3% isoflurane in medical-grade oxygen. After conscious uptake periods of 1 and 2 h, the animals were anesthetized and imaged 2 at a time, side by side in a feet-first prone position as previously described (26).
For biodistribution, the radiotracer (3–3.7 MBq in 100 μL of PBS) was injected into the tail vein, followed by conscious uptake periods of 1 and 2 h (68Ga-1), 2 and 4 h (68Ga-2), or 1, 24, 48, and 72 h (177Lu-1 and 177Lu-2). For blocking studies, the respective peptides 1 and 2 (48 mg/kg, 16 mg/mL solution in PBS) were injected 10 min before the radiotracer. At each time point, the mice (n = 3/time point/radiotracer) were anesthetized and sacrificed, tissues were collected and rinsed with PBS, and the radioactivity was measured with a γ-counter. Radioactivity concentrations were calibrated, decay-corrected, and expressed as percentage injected dose per gram of tissue (%ID/g).
An extended biodistribution study was performed for 177Lu-2. In 100 μL of PBS, 3.7–5.55 MBq were injected into the tail vein of male (n = 4/time point) and female (n = 4/time point) mice, followed by conscious uptake periods of 24 h, 48 h, 72 h, 1 wk, and 2 wk. The dosimetry values for 177Lu-2 were computed using OLINDA/EXM1.1 using a female or male model with organ mass scaling, and effective doses were reported as mSv/MBq.
For the therapeutic efficacy study, mice were randomly chosen and divided into 4 treatment groups: control saline (group 1, n = 5), control peptide 2 (group 2, n = 6), 74 MBq of 177Lu-2 (group 3, n = 10), and 2 × 37 MBq of 177Lu-2 (group 4, n = 7). Tumor volumes at the start of the treatment ranged from 14 to 218 mm3. Group 2 received 20 μg of peptide 2, group 3 received a single dose of 74 MBq (20 μg of peptide 2) on day 14 after tumor implantation (treatment day 0), and group 4 received 1 dose of 37 MBq (10 μg of peptide 2) on days 14 and 21 after tumor implantation (treatment days 0 and 7). Body weights and tumor volumes were measured the day before treatment and twice a week after treatment throughout the study.
Statistical Analysis
All statistical data are reported as mean ± SD. Paired, 2-tailed Student t tests were used to evaluate statistical significance, with a P value of less than 0.05 being considered statistically significant.
RESULTS
Synthesis of 1 and 2 and Respective natGa and natLu Analogs
Peptides 1 and 2 were synthesized by solid-phase peptide synthesis and obtained in high purity (>98%) after high-performance liquid chromatography purification, and natGa-1, natGa-2, natLu-1, and natLu-2 were obtained in at least 98% purity. The analytic data are provided in Supplemental Section 2. natGa-1, natGa-2, natLu-1, and natLu-2 were used in an ELISA for half-maximal inhibitory concentration determination and as reference standards for high-performance liquid chromatography coinjection to confirm the identity of the 68Ga- and 177Lu-labeled peptides.
Radiochemical Synthesis
68Ga-1 and 68Ga-2 were obtained in at least 98% radiochemical purity after semipreparative high-performance liquid chromatography purification. 177Lu-1 and 177Lu-2 were obtained in at least 97% radiochemical purity.
Half-Maximal Inhibitory Concentration Determinations
The half-maximal inhibitory concentration of natGa-1, natGa-2, natLu-1, and natLu-2 for integrin αvβ6 determined by ELISA were 33.2 ± 1.5 nM, 37.2 ± 3.5 nM, 50.0 ± 4.4 nM, and 29.0 ± 0.6 nM, respectively. The binding affinities for integrin αvβ3 were more than 100 μM for all peptides.
Cell Binding, Internalization, and Efflux
For 68Ga-1, 16.1% ± 0.3% of total radioactivity bound to the BxPC-3 cells, of which 71.5% ± 0.6% internalized into the cells. For 177Lu-2, 31.5% ± 0.6% bound, of which 72.3% ± 1.0% internalized, and minimal efflux was observed for 177Lu-2 at 1 h (∼20% of internalized radioactivity) (Fig. 2).
Cell binding and internalization of 68Ga-1 and 177Lu-2 at 1 h to BxPC-3 cells at 37°C, and percentage radioactivity of 177Lu-2 retained in BxPC-3 cells after internalization. Internalization is expressed as fraction of total radioactivity (n = 3/radiotracer; 1 h of incubation).
Albumin Binding
Albumin binding of 177Lu-2 was 54.4% ± 2.8% and 58.1% ± 0.4% for mouse and human serum, respectively, compared with 18.1% ± 0.2% and 16.2% ± 0.4%, respectively, for 177Lu-1.
Serum Stability
68Ga-1 and 68Ga-2 were 100% intact at 2 h in both mouse and human serum. At 24 h, 177Lu-1 and 177Lu-2 were 72% and 78% intact, respectively, in mouse serum and at least 97% intact (both) in human serum.
In Vivo Imaging and Biodistribution
Imaging and Biodistribution of 68Ga-1 and 68Ga-2
Imaging and biodistribution were performed in the subcutaneous BxPC-3 tumor model at 1 and 2 h after injection for 68Ga-1 and at 2 and 4 h after injection for 68Ga-2 (Fig. 3; Supplemental Table 1). Uptake and retention in the tumor were evident at all time points for both 68Ga-1 and 68Ga-2. However, whereas 68Ga-1 cleared from key organs such as the kidneys by 2 h, uptake of 68Ga-2 continued to trend upward. Tumor uptake was 2.6% ± 0.8% at 1 h and 2.0% ± 0.6% at 2 h after injection for 68Ga-1 and 9.4% ± 1.9% at 2 h and 10.4% ± 2.6% at 4 h after injection for 68Ga-2.
(A) Representative transaxial (top) and coronal (bottom) PET/CT cross sections of mice bearing BxPC-3 tumors (arrows) obtained at 2 h after injection. Both images are presented on same scale. Red is PET, and gray is CT. (B–D) Biodistribution showing uptake (%ID/g) of 68Ga-1 (B) and 68Ga-2 (C) in selected organs and αvβ6-positive BxPC-3 tumors (n = 3/group/time point) and tumor-to-tissue ratios 2 h after injection (D). B = bladder; K = kidney; H = heart; int = intestines; lg = large; sm = small.
Renal uptake of 68Ga-1 was 23 ± 3 %ID/g at 1 h, dropping to 14 ± 4 %ID/g at 2 h (P = 0.032). For 68Ga-2, renal uptake was 20 ± 3 %ID/g at 2 h, increasing to 26 ± 2 %ID/g at 4 h (P = 0.039). As depicted in Figure 3D, tumor-to-organ ratios at 2 h after injection for 68Ga-1 and 68Ga-2, respectively, were 0.75 ± 0.23 and 0.88 ± 0.25 for stomach, 1.6 ± 0.5 and 1.3 ± 0.3 for small intestine, 0.98 ± 0.26 and 1.2 ± 0.3 for large intestine, 20 ± 7 and 6 ± 0.9 for liver, 21 ± 2 and 7.2 ± 2.5 for pancreas, and 22 ± 3.9 and 1.1 ± 0.2 for blood. Collectively, these data suggest that 68Ga-1 has the most favorable biodistribution properties for a PDAC imaging agent, clearing rapidly from key organs while being retained by the tumor.
Biodistribution of 177Lu-1 and 177Lu-2
Uptake and retention in the BxPC-3 tumor were at least 4-fold greater for 177Lu-2 than for 177Lu-1, with 177Lu-2 remaining at 5 ± 0.8 %ID/g at 72 h (Figs. 4 and 5; Supplemental Tables 2 and 3). Both 177Lu-1 and 177Lu-2 demonstrated washout from key organs over time, resulting in tumor-to-kidney ratios for 177Lu-1 of 0.08 ± 0.05 at 1 h and 0.14 ± 0.05 at 72 h; for 177Lu-2, the ratios were 0.22 ± 0.05 at 1 h and 0.60 ± 0.02 at 72 h (Fig. 5B). For the same time points, tumor-to-stomach ratios were, respectively, 0.31 ± 0.06 and 0.87 ± 0.28 for 177Lu-1 and 0.50 ± 0.08 and 1.16 ± 0.30 for 177Lu-2 (Fig. 5C). Likewise, tumor-to-pancreas ratios were 5 ± 3 and 9 ± 1.4 for 177Lu-1 and 5 ± 0.06 and 16 ± 5 for 177Lu-2 (Fig. 5D); tumor-to-large intestine ratios were 0.31 ± 0.02 and 1.74 ± 0.39 for 177Lu-1 and 0.77 ± 0.25 and 1.77 ± 0.26 for 177Lu-2. Collectively, these data suggest that 177Lu-2 has the most favorable pharmacokinetics to advance to therapeutic efficacy studies.
Biodistribution showing uptake (%ID/g) of 177Lu-1 (A) and 177Lu-2 (B) in selected organs and αvβ6-positive BxPC-3 tumors (n = 3/group/time point). Lg = large; sm = small.
Uptake of 177Lu-1 and 177Lu-2 in BxPC-3 tumor (A) and tumor-to-organ ratios for kidney (B), stomach (C), and pancreas (D) derived from biodistribution data.
Blocking Studies
Preadministration of the respective blocking peptide (1 or 2) 10 min before administration of 68Ga-1, 177Lu-1, or 177Lu-2 resulted in reduced tumor uptake. Tumor uptake dropped from 2.6 ± 0.8 %ID/g (unblocked) to 0.27 ± 0.02 %ID/g for 68Ga-1 (1 h, −86% change); for 177Lu-1, tumor uptake dropped from 1.2 ± 0.2 %ID/g to 0.3 ± 0.02 %ID/g (1 h, −75% change); and for 177Lu-2, tumor uptake dropped from 7.2 ± 3.0 %ID/g to 4.1 %ID/g (4 h, −42% change; Supplemental Figs. 1–3).
Therapeutic Efficacy
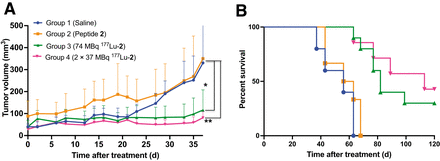
A significant delay in tumor growth was observed in treatment groups 3 (74 MBq 177Lu-2) and 4 (2 × 37 MBq 177Lu-2) versus control groups 1 and 2 (Fig. 6A). All mice in groups 1 and 2 had met the endpoint criteria (tumor ≥ 2 cm in any direction or ulceration) by days 63 and 68 from the start of treatment, respectively. In contrast, the mice in groups 3 and 4 had 30% and 43% survival rates, respectively, at the end of the study (120 d; Fig. 6B). The median survival was 56 d for mice in groups 1 and 2, versus 82 d for group 3 and 113 d for group 4. No treatment-related adverse effects (weight loss or signs of distress) were evident during the course of the study (Supplemental Fig. 4).
Therapeutic efficacy of 177Lu-2 in mice bearing αvβ6-positive BxPC-3 tumors, as determined by tumor growth (average tumor volume ≤37 d after treatment) (A) and survival data (B). *P = 0.0077. **P = 0.0064.
Dosimetry
The highest estimated dose, as expected from the imaging and biodistribution data, was to the kidneys. On the basis of the extrapolated values obtained in OLINDA, the effective dose from 177Lu-2 to the kidneys would be 1.31 mSv/MBq and 1.14 mSv/MBq for a woman and man, respectively (Supplemental Tables 4 and 5).
DISCUSSION
There has been increasingly rapid growth in the field of radiotheranostics, fueled in part by the many successful clinical outcomes, significant investments by the pharmaceutical industry, and recent regulatory approvals (27). Numerous novel radiotheranostics for a wide range of clinically relevant targets are now under investigation (28). Here, we describe the development of peptide-based radiotheranostics to target the integrin αvβ6, an epithelium-specific receptor that has been identified as a relevant molecular target for both the detection and the treatment of cancer (29). Several αvβ6-targeting imaging agents have demonstrated utility in the clinic for a range of carcinomas, including breast, colon, head and neck, lung, and pancreas (16–19). In view of the functional relevance of the integrin αvβ6, it is anticipated to become an important target for future radiotheranostics (30).
Building on over a decade of research and development of αvβ6-targeted peptides for imaging by our group, we designed and report here a radiotheranostic strategy using 68Ga for PET imaging and 177Lu for both treatment and imaging with SPECT. We developed DOTA-5G (1), a peptide with high affinity and selectivity for the integrin αvβ6 and, with the goal to increase tumor uptake and reduce renal retention, further incorporated a 4-(p-iodophenyl)butyryl–containing ABM—based on prior literature showing favorable pharmacokinetics for targeting ligands such as folates, octreotides, and PSMA-targeting phosphoramidates (31–33)—to yield DOTA-ABM-5G (2). Given the notable presence of the integrin αvβ6 in PDAC, we evaluated 1 and 2 in a pancreatic mouse model (11,34,35).
natGa-1, natGa-2, natLu-1, and natLu-2 demonstrated high affinity and selectivity for the integrin αvβ6, similar to our previously published αvβ6-targeting peptides (23,26). Tumors were clearly detected with 68Ga-1 and 68Ga-2 by PET (Fig. 3A) and showed uptake similar to other 68Ga-peptides reported in the literature (19,36). Both 68Ga-1 and 68Ga-2 show predominantly renal excretion, as commonly reported for radiolabeled peptides (37,38). Compared with 68Ga-2, 68Ga-1 was cleared rapidly from the blood and other nontarget tissues, including healthy pancreas (Fig. 3D), resulting in improved contrast. These data reaffirmed our previous reports that although the inclusion of an ABM into targeting ligands increases circulation and tumor uptake, this might not be desirable for short-lived (diagnostic) radioisotopes such as 68Ga (39) or 18F (23). Overall, 68Ga-1 compared favorably to other noteworthy examples, including 68Ga-cycratide (36), 68Ga-DOTA-SFITGv6 (16), and 68Ga-trivehexin (19), and was therefore chosen as the clinical candidate for PET imaging.
In vitro studies showed αvβ6-targeted cell binding and internalization with minimal efflux of the 177Lu-2 from cells. 177Lu-2 also demonstrated good stability (>97% at 24 h) in human serum. Compared with 177Lu-1, the addition of the ABM expectedly resulted in longer circulation of 177Lu-2, which, in combination with the long half-life of 6.7 d for 177Lu, was highly beneficial as demonstrated by the biodistribution studies (Fig. 5). Lu-2 was rapidly taken up by and retained in the tumor over the 72-h window, along with significant washout from all key organs over this time frame. By the 72-h time point, only the kidneys showed higher uptake of 177Lu-2 than the tumor; notably, no renal clearing agents were used in this preclinical study. To further mitigate the high kidney activity, other complementary approaches such as coinjection of positively charged amino acids such as lysine and arginine and pretargeting will be explored. Biodistribution data were extrapolated to obtain estimated human dosimetry using OLINDA/EXM1.1, showing the highest dose to be to the kidney—as expected from the imaging and biodistribution data—at 1.31 mSv/MBq (female) and 1.14 mSv/MBq (male). These values are considerably lower than those reported for other 177Lu-compounds currently under investigation in the clinic (e.g., CTT1403, an agent for the treatment of prostate cancer, was reported as 5.18 mSv/MBq (40)). For 177Lu-2, the estimated dose would equate to approximately a 1.21-Gy (female) and a 1.05-Gy (male) dose to the kidneys, based on a 925 MBq (25 mCi) starting injected dose for our proposed clinical trial, and is significantly lower than the current 23-Gy threshold.
On the basis of the in vitro and in vivo data, it was hypothesized that significant tumor killing could be achieved from selective uptake and retention of 177Lu-2 in αvβ6-positive cells; therefore, the therapeutic efficacy of 177Lu-2 was evaluated in the BxPC-3 tumor xenograft model. Two treatment doses were tested, a high single dose of 74 MBq and a fractioned dose of 2 times 37 MBq, 7 d apart. Both cohorts showed significantly increased survival above the control groups. While all mice in the control groups had reached endpoint criteria by day 68, the mice in the treatment groups had 80%–86% survival rates at that time point and showed 1.5- to 2.0-fold increased median survival times compared with the controls. Importantly, mice in both treatment groups maintained a healthy weight during the study, confirming no adverse effects from either the single dose (74 MBq) or the fractionated double dose (2 × 37 MBq) of 177Lu-2 (Supplemental Fig. 4). These results were highly encouraging and were contrary to the recently published data by Huynh et al., who reported severe weight loss and death due to kidney toxicity within the first 14 d in mouse cohorts receiving a 37 MBq dose of 177Lu-IBA-DOTA-(PEG28)2-A20FMDV2 (35).
Overall, the relevance of integrin αvβ6 in cancer, the high binding of 177Lu-2 to the integrin αvβ6, and its excellent stability in human serum, along with the therapeutic efficacy and favorable estimated dosimetry, collectively suggest the utility of 177Lu-2 as a therapeutic. 177Lu-2 was therefore selected as the treatment candidate for our first-in-humans radiotheranostics study alongside 68Ga-1 as the imaging agent (NCT04665947).
CONCLUSION
68Ga-1 and 177Lu-2 demonstrated high affinity (low nM) and selectivity for the integrin αvβ6 in both in vitro assays and in vivo mouse models, were rapidly internalized, and were stable in human serum. Both radiotracers showed favorable pharmacokinetics in preclinical studies, with predominantly renal excretion and good tumor-to-organ ratios. Favorable human dosimetry data calculated from the murine biodistribution data for 177Lu-2 suggest the potential for this treatment. On the basis of these data, a first-in-humans study in patients with locally advanced or metastatic pancreas cancer is under way.
DISCLOSURE
This work was supported by Stand Up To Cancer and Lustgarten Foundation Pancreatic Cancer Collective (PCC) New Therapies Challenges (SU2C-AACR-PCC-06-18). Sven Hausner is a coinventor of intellectual property related to 1 and 2. Julie Sutcliffe is founder and chief executive officer of, and holds ownership interest (including patents) in, Luminance Biosciences, Inc., and is a coinventor of intellectual property related to 1 and 2. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Can 68Ga- and 177Lu-radiolabeled integrin αvβ6-targeting peptides detect and treat αvβ6-positive cancers?
PERTINENT FINDINGS: 68Ga-radiolabeled integrin αvβ6-targeting peptides could detect αvβ6-positive tumors, and the 177Lu-radiolabeled αvβ6-targeting peptide 177Lu-2 demonstrated therapeutic efficacy in a pancreas cancer mouse model.
IMPLICATIONS FOR PATIENT CARE: This 68Ga-/177Lu-radiolabeled αvβ6-targeting theranostic pair holds significant promise for patients with locally advanced or metastatic pancreas cancer or other αvβ6-positive solid tumors.
ACKNOWLEDGMENT
We thank Charles Smith and Sarah Tam of the Center for Molecular and Genomic Imaging at the University of California Davis for their technical support.
Footnotes
Published online Oct. 7, 2022.
- © 2023 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication August 5, 2022.
- Revision received September 28, 2022.