Visual Abstract
Abstract
Our objective was to assess the diagnostic value of the sentinel node (SN) procedure for lymph node staging in primary intermediate- and high-risk prostate cancer patients with node-negative results on prostate-specific membrane antigen PET/CT (miN0). Methods: From 2016 to 2022, 154 patients with primary, miN0 PCa were retrospectively included. All patients had a Briganti nomogram–assessed nodal risk of more than 5% and underwent a robot-assisted SN procedure for nodal staging. The prevalence of nodal metastases at histopathology and the occurrence of surgical complications according to the Clavien–Dindo classification were evaluated. Results: The SN procedure yielded 84 (14%) tumor-positive lymph nodes with a median metastasis size of 3 mm (interquartile range, 1–4 mm). In total, 55 patients (36%) were reclassified as pN1. A complication of Clavien–Dindo grade 3 or higher occured in 1 patient (0.6%). Conclusion: The SN procedure classified 36% of patients with miN0 prostate cancer with an elevated risk of nodal metastases as pN1.
The presence of lymph node (LN) metastases has a great impact on the prognosis and management of prostate cancer (PCa) (1). Therefore, nodal sampling is recommended for primary PCa patients with a nomogram-assessed risk of LN metastases of more than 5% undergoing radical prostatectomy (2). The gold standard for LN staging in PCa is an extended pelvic LN dissection (ePLND) (2). As this procedure is associated with morbidity (3), alternative options are being explored. The sentinel node (SN) procedure is a procedure in which the first draining LNs are located, removed, and histopathologically assessed for metastases. By yielding a similar diagnostic accuracy and extending nodal detection to aberrant locations, the SN procedure has proven to be a less invasive alternative to ePLND (4,5) but is still considered experimental because of a lack of high-quality evidence supporting its oncologic efficacy (2).
Most studies assessing the diagnostic value of the SN procedure in PCa nodal staging have been performed on patients staged with conventional imaging (4,6). Due to its superior accuracy in detecting macrometastases, prostate-specific membrane antigen (PSMA) PET/CT has now set the new standard in noninvasive PCa staging (7). An intrinsic limitation of this modality is its inability to accurately detect metastases smaller than 3 mm (8,9). Relying on PSMA-based target identification is therefore prone to missing of micrometastases (10). Thus, patients with node-negative PSMA PET/CT (miN0) may still benefit from an SN procedure.
The aim of this study was to investigate the prevalence of LN metastases at final histopathologic examination in miN0 intermediate- and high-risk PCa patients undergoing SN procedures. The secondary outcome was 90-d Clavien–Dindo surgical complications after the SN procedure.
MATERIALS AND METHODS
This retrospective, single-center study was conducted at The Netherlands Cancer Institute. The institutional review board (IRBdm21-216) approved this retrospective study, and the requirement to obtain informed consent was waived. Patients were included if they had biopsy-proven PCa, a Briganti 2012 nomogram–assessed risk of nodal invasion of more than 5% (11), and no evidence of metastases on preoperative staging PSMA PET/CT and underwent the SN procedure before radiotherapy between 2016 and 2022. Patients were excluded if the primary tumor was not visible on PSMA PET/CT. As all patients opted for primary radiotherapy, none received ePLND.
PSMA PET/CT imaging was performed either at our hospital or at the referring hospital. At our center, PET/CT imaging was performed as previously described (12). All PSMA PET/CT scans were reviewed by an experienced nuclear medicine physician in line with PROMISE (13) and discussed in multidisciplinary meetings.
SN procedures were performed as described previously (6), with injection of the hybrid tracer indocyanine green–99mTc-nanocolloid (14) transrectally in 4 quadrants of the prostate under ultrasound guidance. Subsequently, lymphoscintigrams (15 min and 2 h after injection) and SPECT/low-dose CT (SPECT/CT) were performed (Figs. 1A and 1B). Experienced urologists performed robot-assisted surgery using the da Vinci Si Surgical System (Intuitive Surgical Inc.). Intraoperatively, SNs were localized using a laparoscopic γ-probe (Europrobe 2; Eurorad) and fluorescence imaging with the robot-integrated Firefly camera (Intuitive Surgical; Figs. 1C–1D).
SPECT (A), SPECT/CT (B), intraoperative white light (C), and fluorescence imaging (D) of iliac SN, which harbored 3-mm metastasis on histopathology (pN1).
All specimens were fixed in formalin, embedded in paraffin, sectioned at 2 mm, cut at 3 planes (150-μm intervals), stained with hematoxylin and eosin, and histopathologically examined for metastatic deposits. The CAM5.2 monoclonal antibody was used for immunohistochemical evaluation of PSMA expression in LN metastases measuring at least 5 mm.
Median and interquartile range were reported for continuous variables, and frequency and percentage were reported for categoric variables. Unpaired t tests or Mann–Whitney U nonparametric tests was used to compare continuous variables between pN0 and pN1 patients. χ2 or Fisher exact tests was used to compare discrete variables. All statistical analyses were performed with SPSS statistics, version 27.0 (IBM Corp).
RESULTS
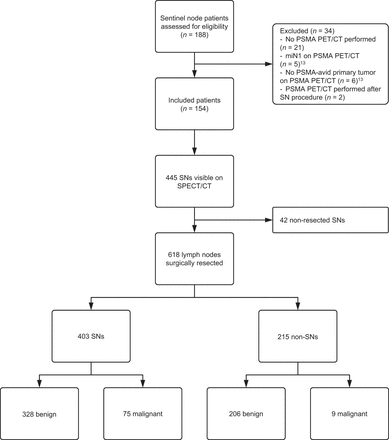
In total, 154 patients met the inclusion criteria and were included in the analysis (Fig. 2; Table 1). Preoperative SPECT/CT highlighted 445 SNs (median, 3 SNs per patient [interquartile range, 2–4]). Patients with unilateral (n = 32) or bilateral (n = 12) nonvisualization on SPECT/CT underwent ipsilateral (n = 11) or bilateral (n = 11) ePLND up to the ureter-vessel crossing, indocyanine green–guided node dissection (n = 20), or a unilateral SN procedure (n = 2). In patients with multiple SNs on SPECT/CT, SNs located in difficult-to-reach anatomic locations (e.g., pararectal or paraaortic) were left in situ (n = 42).
Consolidated Standards of Reporting Trials (CONSORT) diagram.
Patient and Diagnostic Characteristics
In total, 618 LNs were surgically excised (403 SNs and 215 non-SNs), of which 84 (14%) harbored metastases (75 SNs and 9 non-SNs; median metastasis size, 3 mm [Fig. 3]). Nodal metastases were located in the obturator fossa (52%; 44/84), external iliac (39%; 33/84), internal iliac (4%; 3/84), paravesical (2%; 2/84), presacral (1%; 1/84), and pararectal (1%; 1/84) regions. SNs were the only tumor-bearing nodes in 50 (91%) patients.
LN metastasis size distribution.
In total, 55 patients (36%) were upstaged to pN1 on the basis of the outcome of the SN procedure. Sixteen metastases were at least 5 mm, and all showed PSMA expression immunohistochemically.
Only 1 (0.6%) patient had a high-grade complication (Clavien–Dindo ≥ 3; Table 2).
90-D Complications After SN Procedure
DISCUSSION
Using the SN procedure in PCa patients with increased nodal risk and miN0 disease on PSMA PET/CT resulted in upstaging of 55 (36%) patients to pN1, which may alter treatment recommendations. Our institute adjusts the radiation target volume for primary PCa patients according to the histologic outcome of the SN procedure. pN0 patients receive prostate-only radiotherapy, and pN1 patients receive additional pelvic radiotherapy and androgen deprivation therapy intensification. The oncologic benefit of such SN-based radiotherapy field adjustment (15) may also apply to miN0 patients. We believe the high-grade complication rate of 0.6% of the SN procedure justifies its use for nodal staging and subsequent treatment allocation.
The diagnostic value of the SN procedure in miN0 PCa patients treated with radical prostatectomy was previously evaluated (12). The SN procedure detected nodal metastases in 6 (19%) miN0 patients (median metastasis size, 2.0 mm; interquartile range, 1.0–3.0 mm). Building on these results, we demonstrate the diagnostic value of the SN procedure in an expanded cohort of miN0 PCa patients opting for primary radiotherapy.
SN procedures provide a means of mapping the most likely tumor lymphatic drainage, thus allowing detection of metastases and micrometastases that are not yet reached by the vascular supply and might be missed by PSMA targeting (10). In our study, 16 metastases measuring at least 5 mm were missed by PSMA PET/CT. Nearly one third of these positive nodes consisted of multiple micrometastases on histopathology; therefore, although the cross-sectional diameter of the metastasis may be more than 5 mm, the total volume may be too small to be detected by PSMA PET/CT. The median time between PSMA PET/CT and the SN procedure was not significantly different in patients with a metastasis of at least 5 mm and those with a metastasis of less than 5 mm. Therefore, tumor progression is unlikely to explain why macrometastases were missed.
Several limitations of this study are noteworthy. First, different scanning protocols and different PSMA-targeting tracers may have influenced interpretation of PET/CT scans. Second, nodal metastases smaller than 5 mm were not reassessed for PSMA expression. Third, since no ePLND was performed after the SN procedure, we do not know the rate of false-negative SN biopsies. Lastly, oncologic outcomes cannot yet be accurately assessed because many patients received 3 y of androgen deprivation therapy and the current median follow-up is 2 y.
CONCLUSION
Our results demonstrate that the SN procedure detects nodal metastases in more than one third of patients with node-negative PSMA PET/CT. Smaller nodal metastases detected by examination of SNs are readily missed by PSMA PET/CT. Further studies are needed to evaluate the oncologic outcomes of SN-dependent PCa treatment in a prospective setting.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Can SN biopsy improve nodal staging in PSMA PET/CT node-negative PCa patients with an increased risk of nodal invasion?
PERTINENT FINDINGS: SN biopsy upstaged 55 (36%) primary PSMA PET/CT node-negative PCa patients to pN1.
IMPLICATIONS FOR PATIENT CARE: Implementation of SN biopsy in primary PCa improves the detection of metastatic nodes, providing valuable information for further treatment guidance.
Footnotes
Published online Jul. 6, 2023.
- © 2023 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication February 6, 2023.
- Revision received May 31, 2023.