Abstract
Radiation pneumonitis is a rare but possibly fatal side effect of 90Y radioembolization. It may occur 1–6 mo after therapy, if a significant part of the 90Y microspheres shunts to the lungs. In current clinical practice, a predicted lung dose greater than 30 Gy is considered a criterion to exclude patients from treatment. However, contrasting findings regarding the occurrence of radiation pneumonitis and lung dose were previously reported in the literature. In this study, the relationship between the lung dose and the eventual occurrence of radiation pneumonitis after 90Y radioembolization was investigated. Methods: We retrospectively analyzed 317 90Y liver radioembolization procedures performed during an 8-y period (February 2012 to September 2020). We calculated the predicted lung mean dose (LMD) using 99mTc-MAA planar scintigraphy (LMDMAA) acquired during the planning phase and left LMD (LMDY-90) using the 90Y PET/CT acquired after the treatment. For the lung dose computation, we used the left lung as the representative lung volume, to compensate for scatter from the liver moving in the craniocaudal direction because of breathing and mainly affecting the right lung. Results: In total, 272 patients underwent 90Y procedures, of which 63% were performed with glass microspheres and 37% with resin microspheres. The median injected activity was 1,974 MBq (range, 242–9,538 MBq). The median LMDMAA was 3.5 Gy (range, 0.2–89.0 Gy). For 14 procedures, LMDMAA was more than 30 Gy. Median LMDY-90 was 1 Gy (range, 0.0–22.1 Gy). No patients had an LMDY-90 of more than 30 Gy. Of the 3 patients with an LMDY-90 of more than 12 Gy, 2 patients (one with an LMDY-90 of 22.1 Gy and an LMDMAA of 89 Gy; the other with an LMDY-90 of 17.7 Gy and an LMDMAA of 34.1 Gy) developed radiation pneumonitis and consequently died. The third patient, with an LMDY-90 of 18.4 Gy (LMDMAA, 29.1 Gy), died 2 mo after treatment, before the imaging evaluation, because of progressive disease. Conclusion: The occurrence of radiation pneumonitis as a consequence of a lung shunt after 90Y radioembolization is rare (<1%). No radiation pneumonitis developed in patients with a measured LMDY-90 lower than 12 Gy.
Radioembolization is a well-established treatment for primary and metastatic liver malignancies (1). It is defined as percutaneous, transarterial injection (2) of embolic particles (diameter, 20–60 μm) loaded with 90Y or 166Ho. Because hepatic tumors are preferentially fed by the blood supply from the hepatic artery, radioembolization preferentially deposits radioactive microspheres in the peritumoral and intratumoral arterial vasculature through the hepatic artery, relatively sparing normal liver parenchyma (3). Three devices are commercially available: glass 90Y microspheres (TheraSphere; Boston Scientific Corp.), resin 90Y microspheres (SIR-spheres; SIRTeX Medical Limited), and poly-l-lactic acid 166Ho microspheres (QuiremSpheres; Quirem BV). If a significant number of microspheres pass through tumor-associated arteriovenous shunts and lodge in the pulmonary vasculature, a dose-dependent radiation-induced pneumonitis may ensue. Therefore, the presence of significant hepatopulmonary shunting is a relative contraindication for radioembolization. The current approach to radioembolization with respect to radiation pneumonitis is driven mainly by 2 seminal publications (4,5) that have strongly influenced the guidance on lung dose limits after radioembolization. On the basis of clinical evidence from these studies, a lung dose limit of 30 Gy was recommended for a single radioembolization treatment (6) and adopted in the instruction manuals for these devices. For this reason, assessment of the lung shunt fraction (LSF), which is a prediction of the eventual lung mean dose (LMD) after the radioembolization treatment, is paramount before administration of the radioactive particles.
For 90Y, this prediction is performed using 99mTc-macroaggregated albumin (MAA). Despite being the current clinical practice, 99mTc-MAA planar scintigraphy is poor in predicting the dose to the lungs, especially when computing the LSF (LSFMAA) and, consequently, the predicted lung mean dose (LMDMAA). SPECT/CT imaging can improve the LSF computation (7). However, discrepancies between 90Y and 99mTc-MAA particles reduce the predictive value (8,9).
The aim of this study was to assess the occurrence of radiation pneumonitis after 90Y liver radioembolization and perform lung dosimetry on 90Y PET/CT to evaluate the currently assumed lung dose restriction of less than 30 Gy. Although multiple studies on lung dose after 90Y radioembolization have been reported, they all focus on the 99mTc-MAA–based lung dose estimate during the pretreatment phase. Conversely, this study retrospectively quantified the actual dose received by the lungs after 90Y radioembolization, exploiting the potential of posttreatment PET/CT (10) and accurate 90Y dosimetry (11). Knowledge of the actual dose would provide a better insight into the lung dose after 90Y radioembolization and the related occurrence of radiation pneumonitis.
MATERIALS AND METHODS
This single-center, retrospective analysis of all patients treated with 90Y radioembolization between February 2012 and September 2020 was approved by the ethical research committee, and the need for informed consent was waived. Before radioembolization treatment, patient eligibility for treatment was assessed by a 99mTc-MAA injection in the hepatic artery, to assess the intrahepatic distribution and potential extrahepatic deposition of activity (including lung shunting). After the injection, planar γ-camera scintigraphy (to compute LSFMAA) and SPECT/CT (to visually assess extrahepatic depositions) were performed. To assess the treatment outcome, posttreatment 90Y PET/CT was performed on the same day as, or the day after, treatment. LMD after 90Y radioembolization was assessed using the posttreatment 90Y PET/CT.
90Y PET/CT Protocol
Images were acquired on a Biograph mCT or Biograph Vision 600 time-of-flight PET/CT scanner (Siemens Medical Solutions), with 40- and 64-slice CT scanners, respectively. The images were reconstructed using an iterative algorithm including model-based scatter correction, which encompasses a point-spread-function model of the detector response together with time-of-flight information. To correct for attenuation, a low-dose CT scan acquired right after the PET scan was used. Both PET scanners and the reconstruction protocol were validated for 90Y quantitative imaging (12).
99mTc-MAA–Based LMD Predicted
To determine each patient’s eligibility, LMDMAA was calculated as follows: where lung mass is assumed to be 1 kg and 50 [Gy × kg/GBq] is the standard conversion factor for 90Y. LSFMAA was computed as follows:
where lung mass is assumed to be 1 kg and 50 [Gy × kg/GBq] is the standard conversion factor for 90Y. LSFMAA was computed as follows: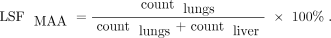
The counts were computed using the geometric mean following standard clinical practice (13). Lungs and liver were delineated on the planar scintigrams by the imaging technicians.
90Y PET–Based LMD
To assess the LMD after treatment, lungs masks were automatically segmented on the CT scans corresponding to the PET scans used for dosimetric purposes, applying a freely available U-net that extracts the right and left lungs separately (14). All masks were visually checked to ensure correct segmentation. Since the right lung was affected by scatter from the liver moving in the craniocaudal direction because of breathing (Fig. 1), only the left lung was considered as representative for computation of the LMD. The 90Y PET–based left LMD (LMDY-90) was computed as follows:
Posttreatment 90Y PET/CT scan of 47-y-old man diagnosed with colorectal cancer. LMD considering both lobes was 61 Gy. 90Y PET image shows activity in right lung (blue contour) due to liver motion in craniocaudal direction and rim field-of-view artifact and leading to right LMD of 100 Gy, which was main contributor to LMD. Left LMD (computed within green contour) was 3 Gy.
The mean activity concentration in the left lung volume of interest (measured in Bq/mL) was computed as the mean of the voxel value (Bq/mL) within the left lung mask. Lung density was assumed to be 2.6e−4 (kg/mm3) (15), whereas 5e−8 (J × s) represents the deposited energy due to β-decay of 1 Bq of injected 90Y activity (16). The mean activity concentration was corrected for 90Y decay considering the time difference between the activity administration and the scanning. Three commonly applied assumptions were adapted for this study. First, the maximal range for 90Y β-particles in tissue is 1.2 cm, which is on the same order of magnitude as the resolution of 90Y PET; thus, it is assumed that the total energy is deposited within the voxel of origin (17). Second, 90Y distributes uniformly in cases of lung shunting. Third, lung density is the same for all patients.
90Y PET–Based LSF
Because LSFMAA is a poor predictor for actual lung shunting, in this work LSF measured using 90Y PET/CT (LSFY-90) was computed as a metric to evaluate differences among tumor type. LSFY-90 was defined as the ratio between the activity in the lungs and the total activity administered, as follows:
As was done for LMDY-90, the mean activity concentration in the lungs was computed as the mean of the voxel value (Bq/mL) within both lung masks. Lung volume was assumed to be the same among all subjects, considering a lung mass of 1 kg and a lung density of 2.6e−4 (kg/mL), previously assumed.
Statistical Analysis
The statistical variables under investigation to characterize radiation pneumonitis were LMDMAA and LMDY-90. When assessing the eventual difference among tumor types or, in the case of hepatocellular carcinoma (HCC) patients, between the presence and absence of portal hypertension and thrombus, we considered LSFY-90 in order to take into account the difference in delivered activity. The normality of distribution was assessed visually and by a Q–Q plot. If variables were not normally distributed, nonparametric tests were used for further analysis.
The Mann–Whitney U test with an α-significance level of 0.05 was used for HCC patients to assess whether the occurrence of thrombus or portal hypertension caused a statistically significant difference in left LMD.
The Kruskal–Wallis H test with an α-significance level of 0.05 was used to determine whether statistical differences existed between different tumor types.
RESULTS
Patient Population
Patients and treatment characteristics are summarized in Table 1. The institutional review board approved this retrospective study and waived the need for informed consent. There were 170 men and 102 women, who underwent a total of 317 90Y radioembolization procedures (mean number of procedures per patient, 1.17; range, 1–5). Most patients were treated for liver metastases of various origins, whereas 25% had HCC. Glass microspheres were used for 200 treatments, and the remaining 117 procedures were performed with resin microspheres. The median administered activity per procedure was 2,278 MBq (range, 277–9,636 MBq) and 1,877 MBq (range, 516–3,245 MBq) for glass and resin microspheres, respectively. The median volume within the PET field of view was 1,713 mL (392–7,851 mL) and 733 mL (range, 80–3,792 mL) for both lungs and the left lung, respectively.
Baseline and Treatment Characteristics
Data Analysis
The median LMDMAA was 3.5 Gy (range, 0.2–89.0 Gy). For 14 patients, LMDMAA was greater than 30 Gy, above which 90Y radioembolization is contraindicated (18). Nonetheless, after clinical consideration by the treating physicians, these patients did undergo 90Y radioembolization treatment.
The median posttreatment LMDY-90 was 1.0 Gy (range, 0.0–22.1 Gy), with 3 cases above 12 Gy. No cases of LMDY-90 above 30 Gy were reported.
The median LSFY-90 was 4.13% (range, 0.27%–39.02%). Overall, according to the Kruskal–Wallis H test, no statistically significant difference existed among tumor types (P = 0.1). However, pairwise comparison among tumor types returned a statistically significant difference between patients with neuroendocrine tumor and patients with colorectal cancer, HCC, or other conditions, with P values of 0.008, 0.010, and 0.022, respectively. Statistically significant P values for LSFY-90 from the pairwise comparison among tumor types are reported in Table 2. A box plot depicting the LSFY-90 per tumor type is shown in Figure 2.
Matrix of Statistical Significance of Differences Between Tumor Types in Terms of LSFY-90
Box plots depicting LSFY-90, together with corresponding median, divided by tumor type. Statistically significant difference was reported between neuroendocrine tumor patients and colorectal cancer patients (P = 0.008), between neuroendocrine tumor and HCC patients (P = 0.010), and between neuroendocrine tumor and patients in group “others” (P = 0.022). CC = cholangiocellular carcinoma; CRC = colorectal cancer; NET = neuroendocrine tumor.
LMDY-90 as a function of LMDMAA is reported in Figure 3. The data suggest that radiation pneumonitis did not occur among subjects with an LMDY-90 below 12 Gy. On the basis of this empiric value and the 30-Gy limit for LMDMAA, the number of true-negative, true-positive, false-negative, and false-positive cases is reported in Figure 3.
Distribution of LMDY-90 as function of corresponding LMDMAA. On the basis of limit of 30 Gy for estimate of absorbed radiation dose to lungs during pretreatment phase and 12 Gy for LMDY-90, below which no radiation pneumonitis cases were reported, subjects were divided into 4 quadrants: true-positive, false-positive, true-negative, and false-negative. According to chosen limits, 12 false-positives were detected. True-positive (red triangles) corresponds to 2 patients who developed radiation pneumonitis, whereas false-negative (blue triangle) corresponds to patient who died of progressive disease before follow-up.
Radiation Pneumonitis
Radiation pneumonitis did not occur in any subject with an LMDY-90 below 12 Gy. Radiation pneumonitis occurred in 2 patients, both of whom were diagnosed with HCC and treated with glass microspheres. The first patient had the highest LMDY-90 (22.1 Gy) of all subjects. This patient had no thrombus or portal hypertension. During the pretreatment work-up, LMDMAA was 89.0 Gy (LSFMAA, 23%), and SPECT/CT showed no evidence of extrahepatic depositions in the upper abdomen. The total administered activity was 7,775 MBq. The second patient had an LMDY-90 of 17.7 Gy in the presence of both portal vein tumor thrombosis and portal hypertension. LMDMAA was 34.1 Gy (LSFMAA, 50%), and SPECT/CT showed no evidence of extrahepatic depositions in the upper abdomen. The total administered activity was 1,300 MBq. Details on this case were previously provided by Alsultan et al. (11).
Another subject, with an LMDY-90 of 18.4 Gy (LMDMAA, 29.1 Gy; LSFMAA, 19%), died of progressive disease 2 mo after treatment, before the evaluation scan could be done.
DISCUSSION
The 30-Gy limit on maximum absorbed dose to the lungs for a single radioembolization treatment was based on clinical evidence from 2 seminal publications (4,5) that have strongly influenced the guidance on lung dose limits after radioembolization. This observational study showed that no patients with an LMDY-90 below 12 Gy developed any lung-dose–related side effects. Of the 14 patients who had an LMDMAA above 30 Gy, 2 developed radiation pneumonitis. However, the 12 other patients with an LMDMAA above 30 Gy did not develop any lung-dose–related side effects, stressing the limitation of using 99mTc-MAA planar scintigraphy in predicting 90Y lung shunts.
Radiation pneumonitis is a rare but potentially fatal side effect of radioembolization. As summarized by Cremonesi et al. (19), there have been various reports of the lung-dose–related side effects of 90Y radioembolization, in an attempt to improve insight on how to define the upper dose limit to the lungs. However, although they all used the same approach to computing the lung dose, namely multiplying the LSFMAA by the administered activity to estimate the LMD, different values for the lung dose above which radiation pneumonitis occurred were found (ranging between 10 and 56 Gy). In line with the 12 false-positive cases reported in this study (Fig. 3), Salem et al. (20) reported 58 patients treated with cumulative or single-treatment lung doses exceeding 30 Gy, based on LSFMAA-derived calculations, who did not develop any radiation pneumonitis or lung toxicity. These findings further underline how 99mTc particles overestimate the actual lung shunt. In contrast, Leung et al. (4) reported radiation pneumonitis in 3 patients with a predicted LMD lower than 30 Gy. However, the absorbed doses taken from the literature were derived without including attenuation correction and thus should be rescaled by an average factor of 0.6 (21). For this reason, a straight comparison with the results of the current study is difficult. In addition, in this study, LMDY-90 was computed on the posttreatment 90Y PET and considering only the left lung as representative of the lung volume. In this study, the same difficulties were found in determining a unique threshold for the 99mTc-MAA–based LMD estimate to avoid radiation pneumonitis, confirming an issue well documented in the literature. As an example, in a multicenter study, Braat et al. (22) reported a patient with an LSFMAA of 3% who developed radiation pneumonitis, whereas the patient with the highest LSFMAA, 33%, did not develop radiation pneumonitis. These contradictory findings in the literature underline the limits of 99mTc-MAA LSF, and consequently lung dose estimate, as predictive of 90Y distribution (9), stressing the need for a more reliable and robust method or particle. In recent years, some alternatives to 99mTc-MAA have been suggested. Kunnen et al. (23) demonstrated in a phantom study that bremsstrahlung SPECT/CT, reconstructed with a Monte Carlo algorithm, can estimate the LSF for a 90Y pretreatment procedure using a theoretically safe 90Y activity of as low as 70 MBq. 166Ho scout microspheres (250 MBq; QuiremScout), already used as scout particles before 166Ho radioembolization, were proposed as a surrogate of 90Y to determine patients’ eligibility, thanks to its imaging possibility (24).
Both patients who developed radiation pneumonitis in this study had HCC. Both cirrhosis and HCC have been associated with increased arteriovenous shunting into the lungs, potentially causing increased lung doses (25). However, significant differences were observed in LSFY-90 only for HCC patients when compared with NET-diagnosed subjects (Fig. 2). In the subgroup of HCC patients only, the presence of either a thrombus or portal hypertension did not play a statistically significant role in LSFY-90, suggesting that these variables might be negligible when assessing the lung-dose–related side effects of 90Y. Conversely, Ward et al. (26), who reviewed 409 patients, reported a low but significant correlation between increased hepatopulmonary shunt fraction (measured using 99mTc-MAA planar scintigraphy) and HCC, hepatic vein tumor thrombosis, and portal vein tumor thrombosis.
Several limitations apply to this study, apart from its retrospective and single-center nature. Considering the 99mTc-based LMDMAA computation, the main limitation is use of a surrogate model applying 99mTc-MAA particles as an approximation to 90Y microsphere distribution. In addition, the lungs and liver were delineated on planar scintigraphy without an anatomic reference and assuming a fixed lung mass of 1 kg. Therefore, women, who have a smaller organ mass (27,28) than men but the same lung shunt, may have received a larger lung radiation dose for the same treatment activity. Like the 90Y PET–based LMDY-90 computation, a constant value for lung density was used. However, as reported by Kappadath et al. (29), use of a constant value might be a limiting factor in an accurate estimate of LMDY-90. Although this study relied on the assumption of lung homogeneity, given that the lungs were not completely within the PET field of view for some datasets, the distribution of microspheres in vivo is heterogeneous (17). The gravitational dependence of alveolar and vascular pressures within the lung causes preferential distribution of blood flow and, in parallel, microspheres to the bases of the lung (30). In addition, microsphere irradiation is microscopically nonuniform (31). However, if radiation pneumonitis occurs, the assumption of a uniform distribution in the lung was visually confirmed by the contrast-enhanced CT scan acquired during follow-up. Regardless, these limitations reflect the current protocols and treatment of patients. Moreover, radiation pneumonitis is a rare side effect of radioembolization, with just 2 cases among the 317 procedures in this study—a number of events too limited for any realistic statistical analysis.
Despite these limitations, a better predictive particle and a new lung dose limit are essential to improve the current general method of selecting patients, avoiding unjustified patient exclusion. Given the proven value of posttreatment 90Y PET/CT (20), more insight should be gained on the actual lung dose delivered than on the predicted one.
CONCLUSION
This observational study showed that radiation pneumonitis did not occur among subjects with a left LMD below 12 Gy, defined on posttreatment 90Y PET/CT. A 99mTc-MAA planar scintigraphy–based estimated cutoff of 30 Gy for lung dose is capricious and, once encountered in pretreatment imaging, should be evaluated with caution to prevent unjustified treatment exclusion.
DISCLOSURE
Martina Stella is employed by the UMC Utrecht under a collaborative grant from the Dutch Research Council (NWO) between UMC Utrecht and Quirem Medical BV. Rob van Rooij and Hugo de Jong have acted as consultants for BTG/Boston Scientific. Arthur Braat has acted as a consultant for BTG/Boston Scientific and Terumo. Marnix Lam has acted as a consultant for BTG/Boston Scientific and Terumo and receives research support from BTG/Boston Scientific and Quirem Medical BV. The Department of Radiology and Nuclear Medicine of the UMC Utrecht receives royalties from Quirem Medical BV. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What is the LMD below which radiation pneumonitis does not occur after 90Y radioembolization?
PERTINENT FINDINGS: This retrospective study showed that all subjects with an LMD below 12 Gy, measured on posttreatment 90Y PET/CT, did not develop radiation pneumonitis.
IMPLICATIONS FOR PATIENT CARE: Our findings suggest reconsideration of the current clinically used upper limit for LMD, 30 Gy.
ACKNOWLEDGMENTS
We thank the IT staff, in particular Marloes van Ijzendoorn, for help and support in providing anonymized data. We express gratitude to Sander Ebbers, who helped with the statistical analysis.
Footnotes
Published online Nov. 12, 2021.
- © 2022 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication September 1, 2021.
- Revision received October 26, 2021.










