Visual Abstract
Abstract
123/131I-metaiodobenzylguanidine (MIBG) scintigraphy has shown a high specificity for imaging pheochromocytoma and paraganglioma, but with low sensitivity because of low spatial resolution. 124I-MIBG PET may be able to overcome this limitation and improve the staging of patients with (suspected) pheochromocytoma. Methods: We analyzed the sensitivity, specificity, and positive and negative predictive values of 124I-MIBG PET in 43 consecutive patients with suspected (recurrence of) pheochromocytoma using histopathologic (n = 25) and clinical validation (n = 18) as the standard of truth. Furthermore, we compared the detection rate of 124I-MIBG PET versus contrast-enhanced (CE) CT on a per-patient and per-lesion basis in 13 additional patients with known metastatic malignant pheochromocytoma. Results: 124I-MIBG PET/CT was positive in 19 (44%) of 43 patients with suspected pheochromocytoma. The presence of pheochromocytoma was confirmed in 22 (51%) of 43. 124I-MIBG PET/CT sensitivity, specificity, and positive and negative predictive values were 86%, 100%, 100%, and 88%, respectively. 124I-MIBG PET was positive in 11 (85%) of 13 patients with malignant pheochromocytoma. Combined 124I-MIBG PET and CE CT detected 173 lesions, of which 166 (96%) and 118 (68%) were visible on 124I-MIBG PET and CE CT, respectively. Conclusion: 124I-MIBG PET detects pheochromocytoma with high accuracy at initial staging and a high detection rate at restaging. Future assessment of 124I-MIBG PET for treatment guidance, including personalized 131I-MIBG therapy, is warranted.
Metaiodobenzylguanidine (MIBG), or iobenguane, is an analog of the adrenergic neurotransmitter norepinephrine and shows uptake in sympathetically innervated tissues such as the heart and salivary glands and tumors that express norepinephrine transporters (1). Because of high accumulation and retention in sympathicomedullary tissue, 123I/131I-MIBG scintigraphy has been used for decades in the imaging of pheochromocytoma and paraganglioma, as well as in many neural crest tumors, with reported sensitivities and specificities of between 80% and 100% (2–4). The sensitivity is hampered considerably by a low spatial resolution, making the assessment of small lesions particularly challenging (5). Because of superior technology and workflows, PET expanded rapidly (6) and is now the standard imaging modality for most cancer entities, including pretherapeutic imaging in patients with neuroendocrine tumors (7) and prostate cancer (8,9). In patients with pheochromocytoma and paraganglioma, somatostatin receptor–targeted PET (10–12) and 18F-flubrobenguane (13) have shown a high specific uptake and a high sensitivity. However, 124I-MIBG PET comes with several potential advantages.
The similarity in its biodistribution to that of 123I-MIBG allows for the translation of current concepts for image interpretation (e.g., SIOPEN [International Society of Paediatric Oncology European Neuroblastoma] and curie), protocols, and medications established by use of 123I-MIBG scintigraphy (14,15).
Furthermore, 124I-MIBG PET potentially combines the high specificity of MIBG imaging with the high sensitivity (5,16) and better quantification of PET tracers (17–19) and thereby addresses major shortcomings of current γ-emitting compounds.
In addition, the long 124I half-life (4.18 d) allows for the assessment of pharmacokinetics by performing imaging and blood tests at multiple time points (18,20–23). Following the theranostic principle, the sister compound 131I-labeled MIBG is applied for radionuclide therapy. In light of the recent Food and Drug Administration approval of 131I-MIBG (24) for the treatment of unresectable, locally advanced, and metastatic pheochromocytoma and paraganglioma, pretherapeutic 124I-MIBG PET can provide valuable information on absorbed doses to the tumor and organs at risk and thereby lay the foundation for personalized dosimetry and activity escalation. The potential of the theranostic pair 124I/131I to improve efficacy and mitigate toxicity has previously been shown in the treatment of patients with differentiated thyroid cancer (20,21,23,25) and in a case report on 124I/131I-MIBG (18).
The aim of this retrospective study was to assess the diagnostic performance of 124I-MIBG PET in patients with suspected pheochromocytoma and in patients with metastatic malignant pheochromocytoma (MMP) before 131I-MIBG therapy. With this intent, we analyzed the optimal imaging time point with regard to tumor uptake, the accuracy in comparison to CT, and the rate of upstaging by use of 124I-MIBG PET in patients with MMP.
MATERIALS AND METHODS
Study Design and Participants
We screened our institutional database for patients who, between March 2005 and March 2017, underwent 124I-MIBG PET/CT for suspected recurrence of adrenal pheochromocytoma and who had sufficient follow-up data for validation. The primary endpoint in these patients was the diagnostic performance, defined as sensitivity, specificity, and negative and positive predictive value. Validation was performed histopathologically or—when histopathology was not available—by an experienced, board-certified endocrinologist on the basis of clinical and laboratory parameters. Secondary endpoints were the diagnostic power of quantitative assessment using SUVpeak for differentiating between patients for whom the suspected pheochromocytoma was confirmed versus those for whom it was ruled out. A subgroup analysis was performed for patients who met the criteria for an indeterminate adrenal mass (size > 10 mm and Hounsfield units [HU] > 10 on non–contrast-enhanced [NC] CT).
For a second analysis, we included patients with known metastatic MMP. The primary metric in this cohort was the lesion detection rate of 124I-MIBG PET using the sum of all detected lesions in conjunction with coacquired contrast-enhanced (CE) CT as the reference standard. The protocol was approved by the Ethics Commission of the University of Duisburg–Essen medical faculty (protocol 20-9656-BO).
124I-MIBG Synthesis
124I-MIBG was manually prepared by the substitution of non–carrier-added 124I-iodine to MIBG. The labeling was performed via copper(I)-assisted isotopic iodine/iodine exchange. 124I-iodine was purchased from PerkinElmer LAS in the form of sodium iodide (124I) as 0.02N NaOH solution. Glacial acetic acid (Merck KGaA) was used as the solvent for all other reactants. Typically, 100–130 MBq of the 124I solution were transferred into a testing tube, 10 μL of a sodium disulfite solution (4 mg/mL) were added, and the mixture was reduced to dryness using a rotating evaporator. A 40-μL volume of a solution containing 100 μg of metaiodobenzylguanidine (Sigma-Aldrich) and 1.5 μL of Cu(I)Cl (0.1 M; Merck KGaA) was added to the residue. The mixture was then heated in the stoppered testing tube at 160°C for 10 min. After the subsequent reduction to dryness, the raw product was resolved in 80–100 μL of hydrochloric acid (0.01 M, aqueous; Sigma-Aldrich) and the volume was injected into a high-performance liquid chromatography system (Waters) for purification. The semipreparative high-performance liquid chromatography was performed isocratically using a LiChroCART 250-4 column (Purospher RP-18, 5 μm; Merck) with radioactivity and ultraviolet detection (254 nm), the eluent being an aqueous solution of NaH2PO4 (1.38 g/L) and acetonitrile, 9:1 (v/v). The product peak (retention time, 13 min; activity channel) was collected in a round-bottom flask, and the volume was reduced to dryness and formulated in 5 mL of phosphate-buffered saline. The volume was collected into a syringe and passed through a 0.22-μm filter (Millex GV; Millipore) directly into a sterile vial (CisBio), yielding 50–70 MBq of formulated 124I-MIBG. Quality control testing of the product was performed using a high-performance liquid chromatography system identical to that for the semipreparative run. Purity was determined to be between 98% and 100%
Imaging Protocol
PET/CT was performed on a Siemens Duo (n = 40, 71%), Siemens Biograph mCT (n = 15, 27%), or Siemens Biograph mMR (n = 1, 2%) at 4 h (n = 26), 1 d (n = 53), 2 d (n = 33), and 4 or 5 d (n = 11) after the administration of a mean activity of 49.8 (interquartile range, 48.3–53.0) MBq of 124I-MIBG. The emission time was 3 min 30 s per bed position for PET/CT and 8 min for PET/MRI. Attenuation correction was performed with the coacquired CT or MRI scan.
Image Analysis
SUVpeak was measured in the 5 lesions displaying the highest tracer uptake for each MMP patient at all imaging time points, with the aim of identifying the imaging time point with the highest average SUVpeak and tumor-to-background ratio, with SUVmean liver as the reference background.
The 124I-MIBG PET/CT images of all preoperative or recurrent pheochromocytomas at this time point were then read by a masked central reader, and pathologic findings were categorized by anatomic region (adrenal gland, bones, or viscera, including distant lymph nodes). The size, SUVpeak, and HU of the adrenal masses on the NC CT images were measured. The CE CT and 124I-MIBG PET/CT images were anonymized separately and read by a masked reader, with at least 2 wk elapsing between reading sessions to avoid recall bias.
Statistical Analysis
The accuracy of 124I-MIBG PET is reported by descriptive statistics. For an indeterminate adrenal mass, separate analyses were performed using the NC CT criteria (>10 HU and >10 mm) versus combined 124I-MIBG PET and NC CT criteria (124I-MIBG PET–positive or adrenal mass >10 HU and >10 mm). The detection rate for patients with MMP was defined as the fraction of 124I-MIBG PET–positive lesions among all lesions for CE CT and 124I-MIBG PET/CT combined. Statistical analysis was performed using SPSS Statistics, version 26 (IBM Corp.). Mann–Whitney U testing was performed to determine the statistical significance of SUVpeak between patients with and without pheochromocytoma. A receiver-operating-characteristic curve, with area under the curve as a metric, was used to determine the predictive potential of SUVpeak for the diagnosis of pheochromocytoma, after the exclusion of 1 patient for whom images were acquired 2 d after the administration of 124I-MIBG. The Youden J statistic was used to identify the optimal cutoff for the diagnosis of pheochromocytoma based on the SUVpeak for all patients and separately for patients with an indeterminate adrenal mass.
RESULTS
Patient Cohort
Fifty-six consecutive patients were eligible. In 43 of 56 patients 124I-MIBG PET was performed for suspected pheochromocytoma because of an elevated catecholamine metabolite level, an unclear renal mass, or the clinical appearance; 4 of these were examined because of suspected recurrence after initial resection. Patient characteristics are given in Table 1. The mean patient age was 51.8 y (range, 20–74 y); 25 patients (58%) were female, and 18 (42%) were male. Three patients (7%) had multiple endocrine neoplasia type IIA, and one (2%) had neurofibromatosis type 1. The mean levels for metanephrine and normetanephrine were 180.9 pg/mL (range, 15–1,377 pg/mL) and 313.8 pg/mL (range, 28–2,358 pg/mL), respectively.
Characteristics of Patients Who Underwent 124I-MIBG PET for Suspected Pheochromocytoma
In 13 patients, 124I-MIBG PET/CT was performed for known MMP. The mean patient age was 50.9 y (range, 17–80 y). Seven (54%) of these 13 patients were female, and 6 (46%) were male.
An overview of the MMP patient characteristics is provided in Table 2.
Characteristics of Patients Who Underwent 124I-MIBG PET for Known Metastatic MMP
124I-MIBG Tumor Uptake
The mean SUVpeak across all lesions was 13.0, 13.3, 12.0, and 9.2 after 4 h, 1 d, 2 d, and 4 or 5 d, respectively. The tumor-to-background ratio at 4 h, 1 d, 2 d, and 4 or 5 d was 1.4, 6.2, 4.9, and 7.6, respectively. Because of the highest tumor SUVpeak and the highest number of available data points 1 d after 124I-MIBG injection, this time point was used for the subsequent accuracy analyses. In 3 patients, for whom 124I-MIBG PET/CT was not performed 1 d after injection, 2-d images were used instead. Figure 1 shows the mean SUVpeak across all measured lesions and the mean tumor-to-background ratio over time.
Simple error bar with 95% CI plotting mean tumor SUVpeak (A) and tumor SUVpeak/tumor SUVmean (B) for all measured lesions in 124I-MIBG PET–positive MMP patients (n = 11) over time.
124I-MIBG PET Accuracy for Suspected Pheochromocytoma
124I-MIBG PET was positive in 19 (44%) of 43 patients with suspected pheochromocytoma and negative in 24 (56%). In 1 of the 19 patients with positive findings on 124I-MIBG PET, a local lymph node metastasis was detected. Twenty-five of the 43 patients had adrenal masses with a density of more than 10 HU on NC CT; in 22 of these, the respective masses were more than 10 mm.
The suspicion of pheochromocytoma was confirmed by the reference standard in 22 (51%) of the 43 patients and ruled out in 21 (49%). Histopathology was available in all cases with confirmed pheochromocytoma and in 3 (14%) of 21 cases in which the presence of pheochromocytoma was ruled out. Of the 22 patients with pheochromocytoma, 21 (95%) had unilateral involvement and 1 (5%) had bilateral involvement. In 18 (42%) of the 43 patients, the presence of pheochromocytoma was excluded on the basis of clinical records and repeated assessment of serum and urine catecholamine metabolites.
An overview of the diagnostic performance of 124I-MIBG PET is provided in Table 3. Imaging was positive in 19 of 22 patients with confirmed pheochromocytoma and negative in 21 of 21 patients in whom the presence of pheochromocytoma was ruled out, resulting in a sensitivity and specificity of 86% and 100%, respectively.
Diagnostic Performance of 124I-MIBG PET, NC CT, and Combined 124I-MIBG PET and NC CT in Patients
In all patients with positive findings on 124I-MIBG PET, pheochromocytoma was histopathologically confirmed, whereas 3 patients with a negative 124I-MIBG PET result would later be diagnosed with pheochromocytoma, leading to a positive predictive value and negative predictive value of 100% and 88%, respectively.
NC CT was available for 36 patients. Of the 25 patients with adrenal masses greater than 10 HU, pheochromocytoma was confirmed in 18 and ruled out in 7; of the 11 patients with adrenal masses less than 10 HU, pheochromocytoma was confirmed in 1 and ruled out in 10, leading to a sensitivity, specificity, positive predictive value, and negative predictive value of 95%, 59%, 72%, and 91%, respectively.
Using an additional size threshold of 10 mm did not affect sensitivity but improved specificity, positive predictive value, and negative predictive value to 76%, 82%, and 93%, respectively.
Combined 124I-MIBG PET and NC CT criteria improved the sensitivity to 100%, specificity to 76%, negative predictive value to 100%, and positive predictive value to 84%.
Pheochromocytomas had a significantly higher SUVpeak than did unaffected adrenal glands (11.8 vs. 3.5, P < 0.001). The area under the curve for the SUVpeak-based identification of pheochromocytoma was 0.88, with the optimal cutoff being 5.4. Subgroup analysis of all patients with adrenal lesions having a density higher than 10 HU showed an area under the curve of 0.90 (sensitivity, 68%; specificity, 100%) for classification into pheochromocytoma versus nonpheochromocytoma. Separate subgroup analyses of all patients with an indeterminate adrenal mass (HU > 10 and size > 10 mm; n = 22) showed an SUVpeak of 8.5 versus 2.9 (P < 0.001), resulting in an area under the curve of 0.90 (sensitivity, 72%; specificity, 100%) with an optimal cutoff of 7.0. Figure 2 gives an overview of the SUVpeak of patients for whom pheochromocytoma was confirmed versus those for whom it was ruled out.
Adrenal SUVpeak for all patients (A) and patients with indeterminate adrenal masses (B) for whom pheochromocytoma was confirmed vs. those for whom it was ruled out.
124I-MIBG PET Detection Rate for Metastatic Pheochromocytoma
In the 13 patients with known MMP lesions, increased focal 124I-MIBG uptake was observed in 11 (85%). Combined CE CT and 124I-MIBG PET detected 173 lesions, of which 166 (96%) showed increased 124I-MIBG uptake, whereas 118 lesions (67%) were detected on standalone CE CT. This led to upstaging from M1a to M1c disease in 1 patient (8%) and migration from oligometastatic disease (>5 tumor sites) (26) in 3 (23%).
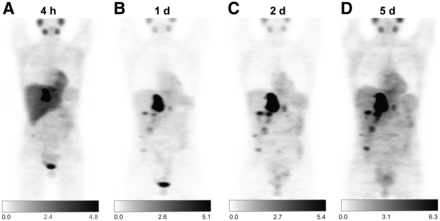
In 5 of these patients, additional 68Ga-DOTATOC PET/CT was performed. 68Ga-DOTATOC–positive/124I-MIBG PET–negative lesions were found in 2 patients, and 68Ga-DOTATOC–negative/124I-MIBG PET–positive lesions were found in 1 patient. In the remainder, 68Ga-DOTATOC PET and 124I-MIBG PET yielded identical results with regard to lesion detection, but tumor-specific uptake was higher on 124I-MIBG PET. Figure 3 shows an example MMP patient with positive 124I-MIBG PET/CT results.
Example patient with metastatic MMP who underwent 124I-MIBG PET/CT before planned radionuclide therapy. At 4 h (A), 1 d (B), 2 d (C), and 5 d (D) after image acquisition, dosimetry-derived activity of 20 GBq of 131I-MIBG was administered, leading to lesion-absorbed doses of between 110 and 320 Gy and progression-free survival of 54 mo.
DISCUSSION
The present study reports high 124I-MIBG PET accuracy at initial staging and a high detection rate at restaging in the—so far, to our knowledge—largest published cohort of patients with (suspected) pheochromocytoma.
Image acquisition 1 d after 124I-MIBG PET demonstrated the highest SUVpeak, and subsequent analyses were performed at this time point.
The reported sensitivity of the present study is comparable to that reported for 123/131I-MIBG scintigraphy studies, and the specificity is close to the higher end of reported values (4,27–30). Sensitivity similar to that of 123/131I-MIBG is unexpected, as the spatial resolution of PET imaging when compared with scintigraphy might translate into a higher accuracy. Interestingly, patients with false-negative 124I-MIBG PET results did not have particularly small pheochromocytomas (3.0, 2.2, and 4.0 cm) when compared with the mean size of all resected pheochromocytomas (3.5 cm), implying that biology and norepinephrine transporter expression, rather than spatial resolution, are critical for lesion detection.
In line with the published literature, NC CT–based assessment has shown a high sensitivity, with only 1 false-negative finding, but at the expense of a low specificity.
Because 124I-MIBG PET enables coacquisition of CE CT, NC CT, or MRI, the diagnostic performance might be improved over that of 123I-MIBG scintigraphy, taking advantage of the high specificity of functional imaging and the high sensitivity of morphologic imaging. Therefore, complementary information from 124I-MIBG PET might be of added value in the workup of unclear adrenal lesions when prior diagnostic imaging is inconclusive and to rule out or confirm metastatic spread before local treatment. In our cohort, visual interpretation of 124I-MIBG images was superior to semiquantitative assessment for the diagnosis of pheochromocytoma. Further studies should assess the potential diagnostic value of 124I-MIBG kinetics in the diagnostic workup of adrenal lesions suggestive of pheochromocytoma. Because of an increase in tumor-to-background ratio over time, images acquired at late time points might aide the differentiation of tumor uptake from physiologic uptake in equivocal lesions.
124I-MIBG PET/CT has also shown a high diagnostic performance in patients with MMP, demonstrating additional lesions compared with standalone CE CT in 7 (54%) of 13 patients, with TNM upstaging in 1 patient (8%) and stage migration from oligometastatic disease (26) to disseminated disease in 3 (23%). The high detection rate may impact patient staging, implementation of local treatment, and response assessment of systemic or local treatment. Detection of additional tumor sites might also prevent locoregional therapies in disseminated disease when little benefit is to be expected.
In light of the 2018 Food and Drug Administration approval of 131I-MIBG radionuclide therapy for MIBG-positive unresectable, locally advanced or metastatic pheochromocytoma and paraganglioma, 124I-MIBG PET/CT furthermore carries great potential for radionuclide therapy planning.
The long half-life or superior quantification of 124I-MIBG PET/CT when compared with 123/131I-MIBG scintigraphy facilitates an improved assessment of uptake intensity and kinetics (18,20,31–34). Pretherapeutic dosimetry of tumor lesions and organs at risk enables personalized dosimetry with the goal of maximizing tumor response while keeping toxicity levels acceptable. The potential of personalized dosimetry for 131I therapy has previously been described in the context of differentiated thyroid cancer (25,35,36), but data on pheochromocytoma are scarce (18,34,37). In our cohort, 85% of patients with known MMP were MIBG-positive. In 2 patients with at least 1 124I-MIBG–negative lesion, intense 68Ga-DOTATOC uptake was observed in all lesions, underpinning the theranostic potential of 68Ga-/177Lu-/90Y-labeled somatostatin analogs (38). Prior small retrospective studies identified radiopeptide as a potential treatment option, leading to response rates of up to 50% and disease control rates of up to 100% (39–42).
Limitations of this study include the retrospective design and the small sample, as well as the absence of an adrenal-specific CT protocol and MR tomography.
CONCLUSION
124I-MIBG PET detects pheochromocytoma with high accuracy at the initial work-up of adrenal masses and at restaging of metastatic disease. Accuracy was similar to that previously reported for 123/131I-MIBG scintigraphy. Future studies on the impact of 124I-MIBG PET on locoregional treatment and personalized 131I-MIBG therapy, as well as a head-to-head comparison with 123/131I-MIBG scintigraphy, are warranted.
DISCLOSURE
Manuel Weber reports fees from Boston Scientific (speakers bureau) outside the submitted work. Ken Herrmann reports personal fees from Bayer, SIRTEX, Adacap, Curium, Endocyte, IPSEN, Siemens Healthineers, GE Healthcare, Amgen, Novartis, and ymabs; personal fees and other fees from Sofie Biosciences; nonfinancial support from ABX; and grants and personal fees from BTG outside the submitted work. Wolfgang P. Fendler reports fees from Calyx (consultant), RadioMedix (image review), Bayer (speakers bureau), and Parexel (image review) outside the submitted work. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What is the diagnostic performance of 124I-MIBG PET in patients with known or suspected pheochromocytoma?
PERTINENT FINDINGS: 124I-MIBG PET has a higher accuracy than NC CT in suspected pheochromocytoma and detects additional lesions in patients with known metastatic pheochromocytoma.
IMPLICATIONS FOR PATIENT CARE: 124I-MIBG PET is a promising imaging technique that can provide information additional to that from cross-sectional imaging and thereby complement the diagnostic workup in patients with known or suspected pheochromocytoma.
Footnotes
Published online Sep. 23, 2021.
- © 2022 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication July 6, 2021.
- Revision received September 8, 2021.