Visual Abstract
Abstract
Prostate-specific membrane antigen (PSMA), overexpressed in prostate cancer, has become a popular target for radionuclide-based theranostic applications in the advanced stages of prostate cancer. We conducted a meta-analysis of the therapeutic effects of PSMA-targeting α-therapy (225Ac-PSMA radioligand therapy [RLT]) in patients with metastatic castration-resistant prostate cancer (mCRPC). Methods: A systematic search was performed using the keywords “mCRPC,” “225Ac-PSMA,” and “alpha therapy.” Therapeutic responses were analyzed as the pooled proportions of patients with more than a 50% prostate-specific antigen (PSA) decline and any PSA decline. Survival outcomes were analyzed by estimating summary survival curves for progression-free survival and overall survival. Adverse events were analyzed as the pooled proportions of patients with xerostomia and severe hematotoxicity (anemia, leukocytopenia, and thrombocytopenia). Results: Nine studies with 263 patients were included in our meta-analysis. The pooled proportions of patients with more than a 50% PSA decline and any PSA decline were 60.99% (95% CI, 54.92%–66.83%) and 83.57% (95% CI, 78.62%–87.77%), respectively. The estimated mean progression-free survival and mean overall survival were 9.15 mo (95% CI, 6.69–11.03 mo) and 11.77 mo (95% CI, 9.51–13.49 mo), respectively. The pooled proportions of patients with adverse events were 62.81% (95% CI, 39.34%–83.46%) for xerostomia, 14.39% (95% CI, 7.76%–22.63%) for anemia, 4.12% (95% CI, 0.97%–9.31%) for leukocytopenia, and 7.18% (95% CI, 2.70%–13.57%) for thrombocytopenia. Conclusion: In our study, around 61% of patients had more than a 50% PSA decline and 84% of patients had any PSA decline after 225Ac-PSMA RLT. The common adverse events in 225Ac-PSMA RLT were xerostomia in 63% of patients and severe hematotoxicity in 4%–14% of patients.
The increasing worldwide incidence of prostate cancer is inevitable because of the increasing number of elderly men (1). The end-stage form of prostate cancer, known as metastatic castration-resistant prostate cancer (mCRPC), is a progressive disease with limited therapeutic options despite androgen deprivation therapy (2). Although several treatment options such as second-generation antiandrogen therapy, taxane-based chemotherapy, and 223Ra are available, a novel treatment approach is necessary given the devastating and lethal course of mCRPC (3).
Prostate-specific membrane antigen (PSMA) is a type II membrane glycoprotein overexpressed in prostate carcinoma, and it has been recognized as a reliable biomarker reflecting disease burden in dedifferentiated and castration-resistant prostate cancer (4,5). Targeting PSMA with diagnostic and therapeutic radionuclide allows the use of the theranostic approach in patients with recurrent or metastatic prostate cancer (6). Recently, the first PSMA-targeting diagnostic radiotracer, 68Ga-PSMA-11, was approved by the U.S. Food and Drug Administration, providing the foundation for PSMA-based theranostics.
PSMA-based radioligand therapy (RLT) with 177Lu, a β-ray–emitting therapeutic radionuclide, has been used in European countries since 2015 for compassionate use in patients with mCRPC (7,8). Since then, several studies have reported positive results when using 177Lu-PSMA RLT (9,10). However, up to 30%–40% of patients were found to be refractory to 177Lu-PSMA RLT during clinical trials and showed hematotoxicity, which limits dose escalation (11).
α-particle–emitting radionuclides, which have higher energy transfer rates and shorter pathlengths, have attracted great attention as an alternative to β-ray–emitting radionuclides for PSMA-based RLT (12). 225Ac has been the first choice as an α-particle–emitting radionuclide in recent experimental PSMA-based RLT for managing patients with mCRPC (13–21). However, given the limited availability of 225Ac coupled with the unstructured clinical setting in these exploratory studies, there is a lack of strong evidence to guide physicians in managing patients with mCRPC using α-particle–emitting RLT. In this context, we conducted a meta-analysis to estimate the therapeutic response, survival outcome, and adverse event of patients with mCRPC who received 225Ac-PSMA RLT.
MATERIALS AND METHODS
Data Search and Study Selection
A systematic search of PubMed, Embase, the Cochrane Library, CINAHL, and Web of Science was conducted on June 10, 2021. The searching keywords were as follows: “metastatic castration-resistant prostate cancer (mCRPC),” “actinium-225 (225Ac) prostate-specific membrane antigen (PSMA),” and “alpha therapy.” Studies that reported the therapeutic response according to the prostate-specific antigen (PSA) evaluation, survival outcome, or adverse event of patients with mCRPC who received 225Ac-PSMA RLT were selected. The search was restricted to publications between 2000 and 2021 written in English. Therapeutic responses were confined to more than a 50% PSA decline or any PSA decline after 225Ac-PSMA RLT. Abstracts, dosimetry/synthesis-related articles, case reports, reviews, editorials, and articles with fewer than 5 patients were not included. When multiple studies were published from the same group, studies with a completely different patient population were included to avoid duplication. Two reviewers independently screened the literature and unanimously selected eligible studies for final inclusion. The protocol of this study was registered in the International Prospective Register of Systematic Reviews (PROSPERO; registration no. CRD42021226139). Institutional review board approval was not required for this meta-analysis because it evaluated published studies.
Data Extraction and Quality Assessment
Publication-related clinical data were extracted from the included articles, and the following information was recorded: first author, year of publication, imaging indication of RLT, number of patients, α-particle–emitting RLT agent, therapeutic dose, therapy cycle, median PSA, median alkaline phosphatase, prechemotherapy (%), prior 177Lu-PSMA (%), prior 233Ra (%), time of PSA evaluation after RLT, therapeutic response, survival outcome, duration of survival follow-up, and adverse events. Two reviewers evaluated each article according to the Newcastle–Ottawa Scale for scoring the quality of nonrandomized studies in meta-analysis (22). This quality scale was categorized into 3 groups (selection, comparability, and outcome) with a perfect score of 8. A maximum of 3 scores could be awarded for selection and outcome, and a maximum of 2 scores could be given for comparability. In cases of discrepancy, 2 reviewers made a consensus decision.
Statistical Analysis
Forest plots were generated to evaluate the effects of 225Ac-PSMA RLT. Therapeutic responses were analyzed as the pooled proportions of patients with more than a 50% PSA decline and any PSA decline, with 95% CIs. Survival outcomes were analyzed by estimating summary survival curves with random effects for progression-free survival (PFS) and overall survival (OS) using the MetaSurv package in R (23). Survival data were read from the Kaplan–Meier curves using the Engauge Digitizer (http://markummitchell.github.io/engauge-digitizer/) (24). Adverse events were analyzed as the pooled proportions of patients with xerostomia and severe hematotoxicity (anemia, leukocytopenia, and thrombocytopenia) with 95% CI. Meta-regression analysis was performed to determine the effect of median PSA, median alkaline phosphatase, prechemotherapy, prior 177Lu-PSMA, and prior 223Ra on the therapeutic response and adverse events. Finally, funnel plots were generated to visually investigate publication bias, and the Egger test was used to evaluate the asymmetry of the funnel plots (25,26). Heterogeneity between the studies (for therapeutic responses and adverse events) was assessed by I2 statistics and χ2 tests (27). The fixed-effects model was used when I2 was not more than 50% and P was at least 0.1 (Cochran Q test), and the random-effects model was used when I2 was more than 50% or P was less than 0.1 (Cochran Q test). Statistical analyses were performed mainly using MedCalc, version 19.1.7, for Microsoft Windows. Comprehensive Meta-analysis Software, Version 3, was used for meta-regression.
A P value less than 0.05 was considered statistically significant.
RESULTS
Study Characteristics
Through electronic database searches, we identified 220 records (Supplemental Tables 1–5; supplemental materials are available at http://jnm.snmjournals.org), and 112 records remained after removing duplicates. Of these, 42 records were excluded on the basis of the title and abstract because of the use of diagnostic radiotracers for PSMA (n = 4), the use of other therapeutic radiotracers (n = 21), in vitro and in vivo preclinical studies (n = 7), and no association with RLT or PSMA (n = 10). After a thorough analysis of the full text of the remaining 70 articles, 61 articles were excluded because of an association with dosimetry, safety, or physics (n = 11); association with synthesis/chemistry (n = 3); being published as case report/review/editorial (n = 44); and inadequate data (n = 3). Finally, 9 studies with 263 patients were included in our meta-analysis (13–19) (Fig. 1). No qualifying study was missed after hand-searching by the reviewers.
Flowchart of study selection process.
Seven of the 9 studies were conducted under a retrospective design (13,15–19,21), and 2 studies were conducted prospectively (14,20). 225Ac-PSMA-617 was administered in 8 studies (13–15,17–21) and 225Ac-PSMA-I&T was used in 1 study (16) as α-particle–emitting RLT agents. The therapeutic dose range per cycle was reported in 3 studies as 1.5–13 MBq (13,16,17), and the total number of treatment cycles ranged from 1 to 8. The median level of baseline PSA was 57.2–331 ng/mL, and the follow-up time for PSA evaluation was 2–6 wk after RLT. Therapeutic responses were reported in all 9 studies involving 263 patients (13–21), and survival outcomes were identified for 200 patients in 6 of the studies (13–15,17,19,20). Adverse events were documented in 8 studies involving 225 patients, which included xerostomia and severe hematotoxicity (13–20) (Table 1). Quality assessment of all 9 studies was performed, and the scores of the Newcastle–Ottawa Scale ranged from 6 to 8 (Table 2).
Baseline Characteristics of Included Studies
Quality Assessment of Included Studies Using Newcastle-Ottawa Scale
Therapeutic Response
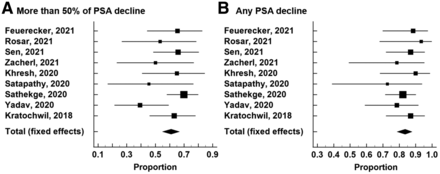
The pooled proportion of patients with more than a 50% PSA decline was 60.99% after 225Ac-PSMA RLT using a random-effects model (95% CI, 54.92%–66.83%), and the I2 statistic was 25.25% (P = 0.219; Cochran Q test). The pooled proportion of patients with any PSA decline was 83.57% after 225Ac-PSMA RLT using a fixed-effects model (95% CI, 78.62%–87.77%), and the I2 statistic was 0.00% (P = 0.844; Cochran Q test) (Fig. 2; Table 3).
Forest plot for therapeutic responses after 225Ac-PSMA RLT: more than 50% PSA decline (A) and any PSA decline (B).
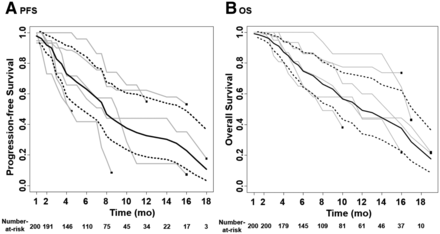
Summary of Therapeutic Responses and Survival Outcomes After 225Ac-PSMA RLT
Survival Outcome
The estimated mean PFS was 9.15 mo (median PFS, 7.78 mo) after 225Ac-PSMA RLT using a random-effects model (95% CI, 6.69–11.03 mo), and the I2 statistic was 7.29%. The estimated mean OS was 11.77 mo (median OS, 11.85 mo) after 225Ac-PSMA RLT using a random-effects model (95% CI, 9.51–13.49 mo), and the I2 statistic was 0.00% (Fig. 3; Table 3).
Survival outcome estimation after 225Ac-PSMA RLT: PFS (A) and OS (B).
Adverse Event
The pooled proportion of patients with xerostomia grade 1 or 2 was 62.81% after 225Ac-PSMA RLT using a random-effects model (95% CI, 39.34%–83.46%), and the I2 statistic was 92.04% (P < 0.0001; Cochran Q test). The pooled proportion of patients with anemia grade 3 or 4 was 14.39% after 225Ac-PSMA RLT using a random-effects model (95% CI, 7.76%–22.63%), and the I2 statistic was 59.32% (P = 0.016; Cochran Q test). The pooled proportion of patients with leukocytopenia grade 3 or 4 was 4.12% after 225Ac-PSMA RLT using a random-effects model (95% CI, 0.97%–9.31%), and the I2 statistic was 58.47% (P = 0.018; Cochran Q test). The pooled proportion of patients with thrombocytopenia grade 3 or 4 was 7.18% after 225Ac-PSMA RLT using a random-effects model (95% CI, 2.70%–13.57%), and the I2 statistic was 58.83% (P = 0.017; Cochran Q test) (Fig. 4; Table 4).
Forest plot for adverse events after 225Ac-PSMA RLT: xerostomia grade 1 or 2 (A), anemia grade 3 or 4 (B), leukocytopenia grade 3 or 4 (C), and thrombocytopenia grade 3 or 4 (D).
Summary of Adverse Events After 225Ac-PSMA RLT
Meta-Regression
Meta-regression analysis for the therapeutic response showed no significant results (Supplemental Table 6). However, the results were significant for adverse events in terms of median PSA (leukocytopenia), median alkaline phosphatase (xerostomia and leukocytopenia), prechemotherapy (anemia and thrombocytopenia), prior 177Lu-PSMA (leukocytopenia), and prior 223Ra (leukocytopenia) (Table 5).
Results of Meta-Regression Analysis for Adverse Event
Publication Bias
Visual investigation of the funnel plots showed no evidence of publication bias for the therapeutic responses and adverse events of 225Ac-PSMA RLT. Egger tests also demonstrated no evidence of funnel plot asymmetry (Fig. 5; Supplemental Fig. 1).
Funnel plot and Egger test for publication bias assessment: more than 50% PSA decline (A) and xerostomia grade 1 or 2 (B).
DISCUSSION
We investigated the effects of 225Ac-PSMA RLT in patients with mCRPC through a meta-analysis. Around 61% of patients achieved more than a 50% PSA decline, and 84% of patients demonstrated any PSA decline after 225Ac-PSMA RLT. The estimated mean PFS and mean OS were approximately 9 and 12 mo, respectively. Xerostomia grade 1 or 2 was observed in 63% of patients, and severe hematotoxicity was noted in approximately 4%–14% of patients.
In comparison with β-ray–emitting radionuclides, α-particle–emitting radionuclides offer several theoretic advantages (12,28). First, the relatively short range of penetration allows the selective killing of targeted tumor tissues while minimizing unwanted damage in the surrounding normal tissues. Second, higher-linear-energy transfer delivers intensive radiation to cancer cells, resulting in more effective DNA strand breakage and reducing the development of treatment resistance.
According to the Prostate Cancer Clinical Trials Working Group 3, the response to therapy of mCRPC patients should be assessed on the basis of PSA changes, and the commonly defined parameter is more than a 50% PSA decline (29). In our study, 61% (95% CI, 55%–67%) of patients showed more than a 50% PSA decline, which is higher than the response in a previous meta-analysis for 177Lu-PSMA RLT (46%; 95% CI, 40%–53%) (30) and a previous phase 2 clinical trial of 177Lu-PSMA-617 (57%) (31). As survival is an important marker in mCRPC patients, the secondary outcomes of our study were PFS and OS after 225Ac-PSMA RLT. The median PFS (8 mo) and median OS (12 mo) in our study were similar to those (11 mo and 14 mo, respectively) in a previous meta-analysis of 177Lu-PSMA RLT (30).
Despite the encouraging therapeutic response and survival of patients who received 225Ac-PSMA RLT, dose reduction or discontinuation of the therapy is often required (32). Xerostomia is a major adverse event in 225Ac-PSMA RLT (33), and our results revealed an incidence rate of 63% (95% CI, 39%–83%). A study highlighted the beneficial effects of sialendoscopy with steroid injection on salivary gland function after 225Ac-PSMA RLT (34); however, it is an invasive procedure. Another study suggested that 225Ac-PSMA/177Lu-PSMA tandem therapy could improve salivary gland function (17). Therefore, more techniques are needed in addition to 225Ac-PSMA RLT to protect salivary gland function (35). In a previous phase 2 clinical trial of 177Lu-PSMA-617, the incidence rate of xerostomia grade 1 or 2 was 87%, which is similar to the incidence rate (63%; 95% CI, 39%–83%) in our study (31). Severe hematotoxicity is another common adverse event of 225Ac-PSMA RLT in previous studies (36), and our study showed anemia grade 3 or 4 in 14% of cases (95% CI, 8%–23%), leukocytopenia grade 3 or 4 in 4% of cases (95% CI, 1%–9%), and thrombocytopenia grade 3 or 4 in 7% of cases (95% CI, 3%–14%). The incidence rates are similar to those in previous studies of 177Lu-PSMA RLT (10,30,37). According to meta-regression analysis, tumor burden and previous damage to bone marrow and salivary glands might adversely affect the toxicity of 225Ac-PSMA RLT. Future studies should consider tumor burden and previous therapy history. Moreover, patient-based dosimetry is required to reduce adverse events and increase the antitumor activity of 225Ac-PSMA RLT.
There were some limitations in this study. The included studies were few in number and had different patient profiles, and the therapeutic doses and cycles of 225Ac-PSMA RLT were somewhat different. Differences in patient profiles likely contributed to the observed heterogeneity, which limits the generalizability of the pooled outcome estimates beyond the reported studies and requires careful interpretation, especially in the aspect of adverse events. Moreover, patient-based analyses could not be performed because of a lack of data on individual patients. In the future, prospective, randomized, multicenter clinical trials are needed to confirm the effects of 225Ac-PSMA RLT.
CONCLUSION
225Ac-PSMA RLT may be an effective treatment option for patients with mCRPC. Our meta-analysis revealed that approximately 61% of patients (95% CI, 55%–67%) showed more than a 50% PSA decline and that 84% of patients (95% CI, 79%–88%) showed any PSA decline after 225Ac-PSMA RLT. Among mCRPC patients who received 225Ac-PSMA RLT, xerostomia (63% of patients; 95% CI, 39%–83%) was the most common adverse event, followed by severe hematotoxicity (4%–14% of patients; 95% CI, 1%–23%).
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What are the effects of 225Ac-PSMA RLT in patients with mCRPC?
PERTINENT FINDINGS: More than a 50% PSA decline and any PSA decline were observed in about 61% (95% CI, 55%–67%) and 84% (95% CI, 79%–88%), respectively of patients after 225Ac-PSMA RLT. The estimated mean PFS and mean OS were about 9 mo (95% CI, 7–11 mo) and 12 mo (95% CI, 10–13 mo), respectively. Xerostomia was the most common adverse event (63%; 95% CI = 39-83%), followed by severe anemia (14%; 95% CI, 6%–23%), severe leukocytopenia (4%; 95% CI, 1-9%), and severe thrombocytopenia (7%; 95% CI, 3%–14%).
IMPLICATIONS FOR PATIENT CARE: PSMA-targeted α-therapy using 225Ac-PSMA may be a novel therapeutic option for mCRPC patients.
ACKNOWLEDGMENTS
We thank Seonok Kim, Msc (Department of Clinical Epidemiology and Biostatistics, Asan Medical Center) for statistical analysis of survival outcomes.
Footnotes
Published online Sep. 9, 2021.
- © 2022 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication January 26, 2021.
- Revision received August 20, 2021.