Visual Abstract
Abstract
Theranostic isotope pairs have gained recent clinical interest because they can be labeled to the same tracer and applied for diagnostic and therapeutic purposes. The goals of this study were to investigate cyclotron production of clinically relevant 133La activities using natural and isotopically enriched barium target material, compare fundamental PET phantom imaging characteristics of 133La with those of common PET radionuclides, and demonstrate in vivo preclinical PET tumor imaging using 133La-PSMA-I&T. Methods: 133La was produced on a 24-MeV cyclotron using an aluminum–indium sealed target with 150–200 mg of isotopically enriched 135BaCO3, natBaCO3, and natBa metal. A synthesis unit performed barium/lanthanum separation. DOTA, PSMA-I&T, and macropa were radiolabeled with 133La. Derenzo and National Electrical Manufacturers Association phantom imaging was performed with 133La, 132La, and 89Zr and compared with 18F, 68Ga, 44Sc, and 64Cu. In vivo preclinical imaging was performed with 133La-PSMA-I&T on LNCaP tumor–bearing mice. Results: Proton irradiations for 100 µA·min at 23.3 MeV yielded 214 ± 7 MBq of 133La and 28 ± 1 MBq of 135La using 135BaCO3, 59 ± 2 MBq of 133La and 35 ± 1 MBq of 135La using natBaCO3, and 81 ± 3 MBq of 133La and 48 ± 1 MBq of 135La using natBa metal. At 11.9 MeV, 135La yields were 81 ± 2 MBq, 6.8 ± 0.4 MBq, and 9.9 ± 0.5 MBq for 135BaCO3, natBaCO3, and natBa metal. BaCO3 target material recovery was 95.4% ± 1.7%. National Electrical Manufacturers Association and Derenzo phantom imaging demonstrated that 133La PET spatial resolution and scanner recovery coefficients were superior to those of 68Ga and 132La and comparable to those of 89Zr. The apparent molar activity was 130 ± 15 GBq/µmol with DOTA, 73 ± 18 GBq/µmol with PSMA-I&T, and 206 ± 31 GBq/µmol with macropa. Preclinical PET imaging with 133La-PSMA-I&T provided high-resolution tumor visualization with an SUV of 0.97 ± 0.17 at 60 min. Conclusion: With high-yield 133La cyclotron production, recovery of BaCO3 target material, and fundamental imaging characteristics superior to those of 68Ga and 132La, 133La represents a promising radiometal candidate to provide high-resolution PET imaging as a PET/α-therapy theranostic pair with 225Ac or as a PET/Auger electron therapy theranostic pair with 135La.
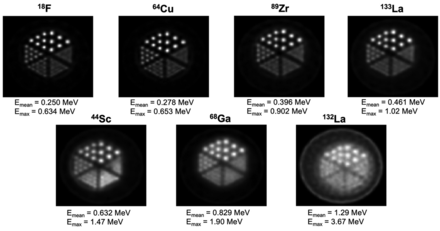
Theranostic pairs in nuclear medicine involve labeling molecular target vectors first with a diagnostic radionuclide, followed by a therapeutic particle–emitting radionuclide (1). Both radionuclides should have similar chemical properties, ideally being isotopes of the same element. Theranostics has strong potential in targeted radionuclide therapy, with a diagnostic positron or γ-emitting radionuclide used in PET or SPECT being paired with a therapeutic radionuclide emitting α-particles, β−-electrons, or Auger electrons (2). Recently introduced 133La (half-life [t½], 3.9 h), 132La (t½, 4.8 h), and 134Ce (t½, 3.2 d)/134La (t½, 6.5 min) PET radionuclides are uniquely suited as theranostic imaging partners for 225Ac (t½, 9.9 d) in targeted α-therapy or with 135La (t½, 19.5 h) in Auger electron therapy (AET) because of their chemical similarity to, and longer half-lives than, the ubiquitous PET radiometal 68Ga (t½, 68 min) (2–7). 225Ac has shown considerable efficacy in clinical trials for treating metastatic cancers (2,8). 132La has been proposed as a theranostic PET imaging surrogate for 225Ac therapy and has displayed in vivo uptake characteristics similar to those of 225Ac (6). However, there are fundamental imaging limitations inherent in 132La because of its high maximum positron emission energy (Emax) and mean positron emission energy (Emean) (Emax/Emean, 3.67/1.29 MeV), which significantly reduces image spatial resolution and contrast compared with other PET radionuclides (e.g., 18F Emax/Emean, 0.634/0.250 MeV; 68Ga Emax/Emean, 1.90/0.829 MeV; 64Cu Emax/Emean, 0.653/0.278 MeV; 44Sc Emax/Emean, 1.47/0.632 MeV), and its high-energy and high-intensity γ-emissions, which are problematic from a dosimetric perspective (3,9). 133La has a lower positron emission energy (Emax/Emean, 1.02/0.461 MeV) than 132La, 68Ga, or 44Sc; energy comparable to 89Zr (Emax/Emean, 0.902/0.396 MeV), and lower energy and lower-intensity γ-emissions than 89Zr, 44Sc, or 132La (3). Here, as outlined in Figure 1, we describe a high-yield cyclotron production method for 133La using natural and isotopically enriched 135BaCO3; phantom measurements comparing fundamental imaging properties of 133La with other PET radionuclides, including 18F, 68Ga, 64Cu, 89Zr, 44Sc, and 132La; and the first (to our knowledge) preclinical PET imaging with 133La. We have chosen to radiolabel PSMA-I&T for imaging prostate cancers.
Experimental overview.
MATERIALS AND METHODS
Chemicals
Table 1 displays the isotopic compositions of 135BaCO3, natBaCO3, and natBa metal. Isotopically enriched 135BaCO3 was obtained from Trace Sciences International. Barium carbonate (99.999% trace metals basis), barium metal (99.99% trace metals basis), American Chemical Society reagent–grade concentrated hydrochloric acid (37%), nitric acid (70%), ammonium hydroxide (28%), and periodic table mix inductively coupled plasma optical emission spectrometry (ICP-OES) elemental standards were obtained from Sigma-Aldrich. Oxalic acid dihydrate (99.5%) was purchased from Fisher Scientific. Aluminum disks were obtained from Michaels, and aluminum foil was purchased from Goodfellow Cambridge. Indium wire was purchased from AIM Specialty Materials. Branched diglycolamide resin was purchased from Eichrom. Eckert and Ziegler Isotopes National Institute of Standards and Technology–traceable γ-ray sources were used for high-purity germanium (HPGe) detector energy and efficiency calibration. Thin-layer chromatography silica gel sheets were purchased from Merck. Water (18 MΩ·cm) was obtained from a MilliporeSigma Direct-Q 3 ultraviolet system. 89Zr was provided by the Washington University Cyclotron Facility. DOTA was purchased from Macrocyclics. Macropa was purchased from MedChemExpress. PSMA-I&T was obtained from ABX Advanced Biochemical Compounds. DCFPyL was synthesized in-house.
Isotopic Composition of Natural and Isotopically Enriched Barium Target Materials
Instrumentation
Activity and radionuclidic purity were assessed using an Ortec GEM35P4-70-SMP HPGe detector running GammaVision software, with dead times below 25%. Elemental purity was assessed using an Agilent Technologies 720 Series ICP-OES. A NEPTIS Mosaic-LC synthesis unit (Optimized Radiochemical Applications) separated 133La from the Ba target solution.
An Eckert and Ziegler AR-2000 radio-thin-layer chromatography imaging scanner quantified the fraction of chelator-bound 133La after reaction. Solid targets were manufactured using a Carver model 6318 hydraulic press and an MTI Corp. 10-mm (internal diameter) EQ-Die-10D-B hardened steel die. A Carbolite 16/610-tube 3-zone furnace was used for 135BaCO3 recovery. X-ray powder diffraction (XRD) patterns were acquired on starting and recovered BaCO3 and intermediate BaC2O4 using a Rigaku Ultima IV x-ray diffractometer to confirm phase identity and purity.
Cyclotron Targeting and Irradiation
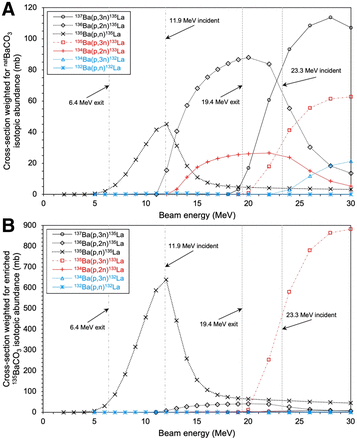
Figure 2 depicts nuclear reaction cross-sections for the 13xBa(p,xn)13xLa reactions of interest for 132/133/135La production from the TENDL 2019 library, weighted for natBa and isotopically enriched 135BaCO3 target material (10). Cyclotron targets were prepared with 150–200 mg of natBa metal, natBaCO3, or enriched 135BaCO3, a roughened aluminum disk (24 mm in diameter, 1.35 mm thick), indium wire (1 mm in diameter), and aluminum foil (125 µm thick) in a manner similar to that previously described (3,11). Aluminum was shown to be an adequate substitute for silver, presenting a lower cost and activation. Target components are shown in Supplemental Figure 1 (supplemental materials are available at http://jnm.snmjournals.org). Targets were irradiated for 5–263 min at 11.9 and 23.3 MeV using an Advanced Cyclotron Systems Inc. TR-24 cyclotron, at proton beam currents of 10 µA incident on the target assembly. Higher energy runs (beam-extracted at 24 MeV, 23.3 MeV incident on target pellets, 20.2 MeV exiting Ba metal, and 19.4 MeV exiting BaCO3) were performed with 200 mg of barium material with the aluminum target cover facing the beam, to maximize 133La production based on TENDL 2019 cross-section simulation data (10). During higher-energy runs, a silver disk was placed behind the target to avoid 13N production from the 16O(p,α)13N reaction. For lower-energy runs (18.2-MeV extraction, 11.9 MeV incident on target pellets, 7.8 MeV exiting Ba metal, and 6.4 MeV exiting BaCO3), performed to maximize 135La production, 150 mg of barium material were used, and the target was installed in reverse with the aluminum disk acting as a degrader to reduce beam energy from 18.2 to 11.9 MeV, as calculated using SRIM (12).
Nuclear reaction cross-section simulation data of proton-induced nuclear reactions on 132/134/135/136/137Ba for 132/133/135La production weighted for natBa isotopic abundance (A) and isotopically enriched 135BaCO3 abundance (B) (10).
Automated 133La Separation and Radiochemical Purity Analysis
133La and BaCO3 were separated using a process with aspects derived from previous studies (3,4). The target was opened by peeling back the aluminum cover and placed in a Teflon (DuPont) dissolution vessel. The vessel was filled with 10 mL of 18 MΩ·cm water and sonicated in an ultrasonic bath for 3 min to dislodge the BaCO3 from the target backing. Target components were removed and rinsed with 5 mL of 18 MΩ·cm water into the vessel, and 5 mL of 3 M HNO3 were added, resulting in a 0.75 M HNO3 reaction mixture that dissolved the BaCO3 in 5 min. This solution was passed through a solid-phase extraction cartridge containing 0.50 g of branched diglycolamide resin (conditioned with 10 mL of 3 M HNO3) and washed with 50 mL of 3 M HNO3 to remove residual barium and other metal impurities, followed by column deacidification with 5 mL of 0.5 M HNO3. Flow rates were kept below 2 mL·min−1 to avoid 133La loss from the resin. 133LaCl3 was eluted using 1 mL of 0.05 M HCl. After passing through the resin, the first 30 mL of process solution were diverted to a collection vial for subsequent BaCO3 recovery. After separation, target components were sonicated in 18 MΩ·cm water for reuse. Radionuclidic and elemental purity of 133LaCl3 was determined by HPGe γ-ray spectroscopy and ICP-OES.
BaCO3 Target Material Recovery
The 30 mL of barium recovery solution were neutralized to pH 6–8 with NH4OH. Ten milliliters of 0.8 M C2H2O4 were added to the recovery solution to precipitate BaC2O4. The solution was passed through a fritted column to trap BaC2O4 and washed with 50 mL of 18 MΩ·cm water. BaC2O4 was removed from the column and then heated to 550°C for 2 h in a sealed tube furnace with an airflow of 20 mL/min to decompose BaC2O4 to BaCO3 while avoiding conversion to BaO (13). Waste gases from decomposition were vented to a fume hood. Recovery was quantified by gravimetric analysis of dried samples and tracked by HPGe γ-spectroscopy using γ-emissions from 135mBa (268 keV; t½, 28.7 h). Samples of purchased BaCO3, precipitated BaC2O4, and recovered BaCO3 were analyzed by XRD to identify the product and evaluate its quality.
Phantom Imaging
Phantom imaging was performed using Derenzo and National Electrical Manufacturers Association (NEMA) image-quality phantoms on an Inveon PET/CT scanner (Siemens Preclinical Solutions), as described by Ferguson et al. (14). The Derenzo phantom, used to investigate image contrast and spatial resolution, consists of sections with rods of varying diameters (0.8, 1.0, 1.25, 1.5, 2.0, and 2.5 mm) that are filled with the radionuclide of interest diluted in 20–30 mL of water. The NEMA phantom, used to investigate image noise, spillover ratio, and recovery coefficient, consists of several fillable sections including two 7.5-mm-diameter cold-air and water cylindric volumes. NEMA and Derenzo phantom scans for 133La, 132La, and 89Zr were acquired in list mode, binned into sinograms, and reconstructed with the default filtered backprojection, ordered-subset expectation maximization, and maximum a posteriori estimation algorithms. Acquisition, data processing, and evaluation followed the same procedure as used by Ferguson et al. (14) for 18F, 64Cu, 68Ga, and 44Sc to enable direct comparison of the different radionuclides’ imaging performance.
Radiolabeling of DOTA, PSMA-I&T, and Macropa with 133La
Similar to techniques in previous studies (3,4), the activity of a 500-µL 133LaCl3 aliquot was measured, and the solution pH was adjusted to 4.5 with 50 µL of NaOAc buffer (pH 9.0). A 100-µL volume of this 133La solution (5–150 MBq) was reacted with 0.1–20 µg of DOTA, PSMA-I&T, and macropa dissolved in 50 µL of 18 MΩ·cm water at 90°C for 30 min. Each solution was analyzed using radio–thin-layer chromatography on silica plates to determine radiochemical purity and incorporation with 0.1 M citric acid buffer as the mobile phase.
Preclinical PET Imaging
Animal studies using LNCaP tumor–bearing male nu/nu nude mice (Charles River Laboratories) were performed according to the guidelines of the Canadian Council on Animal Care and approved by the local Cross Cancer Institute Animal Care Committee. Static PET image scans (20-min duration) of 133La-PSMA-I&T at 60 min after injection were performed on an Inveon PET/CT scanner (Siemens Preclinical Solutions). Blocking experiments were performed using the PSMA-targeting agent DCFPyL. Radiotracer (33–50 MBq of 133La-PSMA-I&T in 80–120 µL of NaOAc/saline) and blocking compound (300 µg of DCFPyL, dosed 5 min beforehand) were injected into the tail vein of isoflurane-anesthetized mice (100% oxygen; gas flow, 1.5 L/min), the mice were placed in a prone position into the center of the field of view, and body temperature was kept constant at 37°C. A transmission scan for attenuation correction was not acquired. The frames were reconstructed using ordered-subset expectation maximization and maximum a posteriori algorithms. No correction for partial-volume effects was applied. The image files were processed using ROVER software (version 2.0.51; ABX GmbH).
Statistical Analysis
All data are given as mean ± SD (n ≥ 3).
RESULTS
Cyclotron Targeting and Irradiation
Average end-of-bombardment activities (n = 3) of 133La and coproduced 135La for 100 µA·min runs (10 µA for 10 min) at 11.9- and 23.3-MeV beam energies with different barium target materials are summarized in Table 2. Irradiating enriched 135BaCO3 at 23.3 MeV resulted in a significant increase in 133La production compared with natBaCO3 and natBa metal. Irradiating recovered natBaCO3 at 23.3 MeV for 100 µA·min yielded 57 ± 1 MBq of 133La and 36 ± 1 MBq of 135La, similar to yields for fresh natBaCO3.
Average Experimental (n = 3) End-of-Bombardment Activities (MBq) and Saturated Yields (MBq/µA) of 133/135La for 100-µA·Min Runs at 11.9- and 23.3-MeV Incident Energies for Different Barium Target Materials
133La Separation and Radiochemical Purity Analysis
Table 3 contains ICP-OES elemental purity results for the 133LaCl3 product. After removal from the reactor after sonification, the aluminum target backing and cover contained no detectable 133La activity. The entire separation took approximately 50 min. Over 92% of decay-corrected 133La was recovered in 1 mL of 0.05 M HCl, and HPGe analysis of the 133LaCl3 product produced with natBaCO3 showed small activities of 131La (t½, 59 min) and 132La (t½, 4.8 h) with no other observed radionuclidic impurities, similar to previous findings (3). 131La and 132La were not observed in 133LaCl3 produced with isotopically enriched 135BaCO3. Elemental purity determined by ICP-OES of 133LaCl3 produced with fresh and recovered BaCO3 target material was superior to 133LaCl3 previously produced with barium metal as described in a previous publication (3).
ICP-OES Analysis (n = 3) of 133LaCl3 Produced with Different Barium Target Materials
Enriched 135BaCO3 Recovery
Figure 3 depicts the decay-corrected fraction of total 135mBa and 135La activity as a function of process volume. The solution was collected in fractions (5 mL for 0–75 mL, 0.5 mL for 75–80 mL) after flowing through the resin, and each fraction was analyzed on the HPGe to quantify 135mBa and 135La activity via their respective 268- and 481-keV γ-emissions. Over 99.7% of decay-corrected 135mBa activity was recovered in the first 6 fractions, with no detectable contributions from additional fractions; therefore, only the first 30 mL of process solution were collected for recovery.
Decay-corrected fraction of initial 135mBa and 135La target activity in solid-phase extraction cartridge eluate as function of process volume.
BaC2O4 formed a white precipitate and was collected by the fritted column. After BaC2O4 thermal decomposition to BaCO3 from heating at 550°C, gravimetric analysis indicated a recovery of 191.1 ± 3.2 mg, which for a 200.3 ± 0.3 mg initial target pellet mass corresponds to a BaCO3 recovery of 95.4% ± 1.7% (n = 3).
Figure 4 depicts the XRD diffractograms acquired for fresh BaCO3, intermediate BaC2O4, and recovered BaCO3 material. Complete XRD diffractogram data are in Supplemental Tables 1–3 and Supplemental Figures 2–4. The absence of unexplained reflections in all 3 patterns, compared with standard reference lines, confirmed the high phase purity of the compounds and the complete conversion of BaC2O4 to BaCO3 (15).
Background-stripped XRD diffractograms of fresh BaCO3, intermediate BaC2O4, and recovered BaCO3.
Phantom Imaging
Figure 5 depicts Derenzo phantom scans with the mean and maximum positron energies of 133La, 132La, and other commonly used PET radionuclides. Derenzo phantom scans acquired with 18F, 64Cu, 89Zr, 133La, 44Sc, 68Ga, and 132La clearly show that lower mean and maximum positron energies improve PET image spatial resolution and contrast. 133La exhibits spatial resolution similar to that of 89Zr, is an improvement over 44Sc and 68Ga, and is superior to 132La.
Derenzo phantom images reconstructed with maximum a posteriori estimation for different PET radionuclides, presented in order of increasing positron emission energy. 18F, 64Cu, 44Sc, and 68Ga data were taken from Ferguson et al. (14).
Figure 6 plots the contrast between the rods and background for each of the 6 triangular segments in the Derenzo phantom and the recovery coefficients as a function of rod size in the NEMA image-quality phantom. Additional comparisons of imaging performance metrics between radionuclides for different reconstruction algorithms are included in Supplemental Figure 5. 133La exhibits contrast similar to that of 89Zr and is superior to 68Ga and 44Sc for larger rod diameters. 132La was not included in the contrast comparison because of the low contrast for each rod diameter. The rods could not be distinguished below 1.25 mm in diameter for the higher-energy positron emitters 44Sc and 68Ga and 1 mm for the lower-energy positron emitters 18F and 64Cu. This blurring is due to the extrinsic scanner resolution, which is significantly impacted by the positron energy and therefore range. The recovery coefficient comparison demonstrates that 133La exhibits favorable performance compared with 68Ga and 132La.
(A) Normalized contrast as function of rod size for different radionuclides in Derenzo phantom. (B) Impact of radionuclide and reconstruction method on measured recovery coefficients in NEMA image-quality phantom. 18F, 64Cu, 44Sc, and 68Ga data were taken from Ferguson et al. (14). 2D = 2-dimensional; 3D = 3-dimensional; FBP = filtered backprojection; MAP = maximum a posteriori; OSEM = ordered-subsets expectation maximization.
Radiolabeling
Radiolabeling was performed at 90°C for 30 min and analyzed with radio–thin-layer chromatography using 0.1 M citric acid buffer as the mobile phase. The 133La-DOTA, 133La-PSMA-I&T, and 133La-macropa complexes remained close to the thin-layer chromatography baseline (Rf, 0.1–0.2), whereas unreacted 133La migrated toward the solvent front (Rf, 0.9–1.0). Titration of 133LaCl3 (n = 3) yielded an apparent molar activity of 130 ± 15 GBq/µmol with DOTA, 73 ± 18 GBq/µmol with PSMA-I&T, and 206 ± 31 GBq/µmol with macropa.
Preclinical PET Imaging
Figure 7 depicts static PET images of LNCaP tumor–bearing mice 60 min after injection of 33–50 MBq of 133La-PSMA-I&T (n = 4). Tumor uptake was significant, reaching an SUVmean of 0.97 ± 0.17 after 60 min. The SUVmean for muscle was 0.05 ± 0.01, resulting in a tumor-to-muscle ratio of 22.4 ± 4.5. Mice predosed with 300 µg of DCFPyL 5 min before 133La-PSMA-I&T injection exhibited significant tumor blocking, with a tumor SUVmean of 0.11 ± 0.01 after 60 min. Most other radioactivity was excreted into the kidneys and urinary bladder.
Representative PET maximum-intensity-projection images at 60 min of 133La-PSMA-I&T with and without predose of DCFPyL in LNCaP tumor–bearing mice. ID = injected dose; MIP = maximum-intensity projection; p.i. = after injection.
DISCUSSION
This study presents cyclotron production of 133La using natural and isotopically enriched barium target material, favorable fundamental PET phantom imaging characteristics of 133La, and the first (to our knowledge) in vivo preclinical PET tumor imaging using 133La-PSMA-I&T.
The new target assembly is well suited to the irradiation and processing of barium metal and BaCO3 target material. Using aluminum instead of silver target backings as used in previous studies (3,11) avoids production of long-lived 107Cd, 109Cd, and 106mAg, thereby strongly reducing overall activation of the target, lowering operator exposure, and enabling rapid reuse. Using the target backing as an intrinsic degrader simplifies and enhances the available range of irradiation energies. The indium wire seal stayed 1–2 mm outside the target beam spot, avoiding activation and formation of radiotin isotopes. Sonicating used target disks in 18 MΩ·cm water allowed repeated reuse to make additional targets, with the same seal and target backing reused over 5 times.
Irradiating enriched 135BaCO3 at 23.3 MeV produced far more 133La than did other target materials, allowing production of clinically relevant 133La activities with significantly shorter irradiation times than using natBa target material. Target separation gave a high 133LaCl3 yield in a 1-mL product volume, ready for radiolabeling.
Recovery of BaCO3 target material demonstrated feasibility for cost-effective recovery of expensive isotopically enriched 135BaCO3. XRD analysis of recovered BaCO3 showed complete conversion of the BaC2O4 intermediate and a pure recovered product, validating target material recovery and highlighting the potential for substantially improved economics with a simple and inexpensive recovery process. Radiolabeling DOTA, PSMA-I&T, and macropa with 133La achieved high apparent molar activities for fresh and recovered BaCO3 target material, similar to radiolanthanum chelation in previous studies (3–5,16).
Using isotopically enriched 135BaCO3 target material permits selective production of 133La and 135La compared with natBa target material. Performing irradiations at energies of 23.3 MeV or higher significantly increases 133La production via the 135Ba(p,3n)133La reaction and reduces 135La production from the 135Ba(p,n)135La reaction, which is ideal for PET imaging applications. Irradiating at 11.9 MeV with enriched 135BaCO3 is ideal for producing large activities of pure 135La for AET. Using these 2 distinct reactions permits production of a variety of 133/135La isotopic blends on a variable-energy cyclotron.
Another production route could use isotopically enriched 134BaCO3 target material to produce 133La via the 134Ba(p,2n)133La reaction. This would enable 133La production on lower-energy cyclotrons because of the 134Ba(p,2n)133La cross-section threshold at 12 MeV as opposed to the 20 MeV threshold for the 135Ba(p,3n)133La reaction. The lower natural isotopic abundance of 134Ba (2.4%) than of 135Ba (6.6%) would result in a higher isotopic enrichment cost. However, this is a compelling option for PET centers with lower-energy cyclotrons because of the 95.4% recovery yield of BaCO3 target material demonstrated in this study.
PET phantom imaging clearly showed that 133La exhibits spatial resolution and contrast superior to those of 44Sc, 68Ga, 132La but similar to those of 89Zr. As expected, lower positron emission energy leads to improved spatial resolution (17) and results in superior image quality for 133La versus 132La, 68Ga, and 44Sc. This superiority is clearly translated to preclinical imaging, as evidenced by high spatial resolution. Even with the lower positron branching ratio of 133La (7.2%) versus other PET radionuclides (96.7% 18F, 88.9% 68Ga, and 41.2% 132La), the LNCaP tumor was clearly defined, reaching an SUVmean of 0.97 ± 0.17 or 3.94 ± 0.68 %ID/g at 60 min after injection. For 68Ga-PSMA-I&T, 4.95 ± 1.47 %ID/g uptake into LNCaP tumors was reported in an ex vivo biodistribution study (18). As discussed previously (3), in vivo studies involving retention and dosing of 133La decay daughter 133Ba would be useful to address this potential limitation; however, as shown by Newton et al. (19), most 133Ba activity could be expected to be excreted within 10 d after injection.
Since lanthanum and actinium are group 3 elements with similar chemical properties, 133La is highlighted as a strong candidate to become a clinical PET imaging surrogate for 225Ac α-therapy, with PET imaging characteristics superior to those of 132La. As established in this study and previously (3), compared with 132La, 133La has superior inherent cyclotron production characteristics, a lower positron energy that translates to a higher spatial resolution, and lower-energy and lower-abundance γ-emissions that would translate to a lower patient and operator dose. These characteristics suggest that 133La represents an attractive candidate for diagnostic PET imaging and treatment monitoring of clinical 225Ac targeted α-therapy and research involving 135La AET.
CONCLUSION
This work demonstrates the strong potential of 133La to serve as a theranostic PET imaging agent with 225Ac targeted α-therapy or 135La AET. The first preclinical in vivo PET imaging studies on LNCaP tumors resulted in high spatial resolution and contrast. Phantom imaging of 133La demonstrated that fundamental PET imaging properties, including spatial resolution, contrast, and recovery coefficient, were superior to those of other PET radiometals such as 68Ga, 44Sc, and 132La and similar to those of 89Zr. With cyclotron production routes capable of generating clinically relevant 133La activities, and with demonstrated feasibility for performing high-yield recovery of expensive isotopically enriched 135BaCO3 target material, 133La appears to be a promising radiometal candidate for high-resolution PET imaging as a PET/targeted α-therapy theranostic pair with 225Ac or a PET/AET theranostic pair with 135La.
DISCLOSURE
The Dianne and Irving Kipnes Foundation supported this work. Bryce Nelson received graduate scholarship funding from Alberta Advanced Education and the Natural Sciences and Engineering Research Council of Canada. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is the positron emitter 133La suitable for in vivo tumor imaging, and how do its production techniques and fundamental imaging characteristics compare with those of other PET radionuclides?
PERTINENT FINDINGS: Phantom imaging showed the PET spatial resolution of 133La to be superior to that of 68Ga, 44Sc, and 132La and comparable to that of 89Zr. Preclinical imaging with 133La-PSMA-I&T in tumor-bearing mice clearly delineated tumors with high spatial resolution. Robust, economical, high-yield cyclotron 133La production was demonstrated using recoverable isotopically enriched 135BaCO3 target material.
IMPLICATIONS FOR PATIENT CARE: This study showed that 133La is a strong candidate to improve patient care by providing PET imaging of tumors as a theranostic pair with 225Ac targeted α-therapy or potential 135La AET.
ACKNOWLEDGMENTS
We thank Jonathan Doupe (Alberta Health Services) for guidance throughout cyclotron production, Samantha Leier (Department of Oncology) for preparing 89Zr for phantom imaging, and Rebecca Funk (Department of Earth and Atmospheric Sciences) for performing XRD data collection.
Footnotes
Published online August 12, 2021.
- © 2022 by the Society of Nuclear Medicine and Molecular Imaging.
Immediate Open Access: Creative Commons Attribution 4.0 International License (CC BY) allows users to share and adapt with attribution, excluding materials credited to previous publications. License: https://creativecommons.org/licenses/by/4.0/. Details: http://jnm.snmjournals.org/site/misc/permission.xhtml.
REFERENCES
- Received for publication April 18, 2021.
- Revision received July 16, 2021.