Visual Abstract
Abstract
With translation of the Drop-In γ-probe, radioguidance has advanced into laparoscopic robot-assisted surgery. Global-positioning-system–like navigation can further enhance the symbiosis between nuclear medicine and surgery. Therefore, we developed a fluorescence-video–based tracking method that integrates the Drop-In with navigated robotic surgery. Methods: Fluorescent markers, integrated into the Drop-In, were automatically detected using a daVinci Firefly laparoscope. Subsequently, a declipseSPECT-navigation platform calculated the Drop-In location within the surgical field. Using a phantom (n = 3), we pursued robotic navigation on SPECT/CT, whereas intraoperative feasibility was validated during porcine surgery (n = 4). Results: Video-based tracking allowed for navigation of the Drop-In toward all lesions detected on SPECT/CT (external iliac and common iliac artery regions). Augmented-reality visualization in the surgical console indicated the distance to these lesions in real time, confirmed by the Drop-In readout. Porcine surgery underlined the feasibility of the concept. Conclusion: Optical navigation of the Drop-In probe provides a next step toward connecting nuclear medicine with robotic surgery.
In the pursuit of precision surgery, robot-assisted approaches are gaining traction (e.g., prostatectomy and lymphatic dissections). An effective minimally invasive approach requires not only well-engineered (robotic) instruments but also precise target definition. Interventional molecular imaging can help achieve this: radioguided surgery is one of the most used image-guided surgery techniques (1). Unfortunately, the use of laparoscopic γ-probes is cumbersome with the robot (2). To overcome these limitations, tethered drop-in γ- and β-probes have been introduced for sentinel lymph node resection (2,3) and prostate-specific membrane antigen–targeted resection (4,5). These robot-tailored modalities allow the surgeon to autonomously position the detector to localize lesions during surgery. In areas with great anatomic complexity, road maps provided by preoperative imaging (SPECT/CT or PET/CT) are considered a critical tool to provide insight on the number and location of surgical targets (6). Unfortunately, during robotic procedures, real-time registration of such preoperative imaging information into the surgical view is challenging.
SPECT-based navigation of γ-probes could translate road maps created at nuclear medicine to the surgical theater (7). Unfortunately, traditional tool-tracking technologies cannot be used with a drop-in probe, whose tethered nature prohibits tracking with external optical tracking systems, and the metal parts of the robot setup degrade the accuracy of electromagnetic tracking systems (8,9). Alternatively, video-based tracking concepts have been pursued (8,9). Use of patterned-surface markers, which can be segmented from a laparoscopic video feed using traditional white-light imaging, has been explored for tethered ultrasound and γ-probes (10,11). For such a tracking method, a direct line of sight has to be maintained between the drop-in probe and the laparoscope. However, Wild et al. have proposed use of fluorescence surface markers, with which a direct line of sight is less easily impaired by smoke, water, or blood and which can be used in combination with fluorescence guidance (12). In combination with multicolor fluorescence imaging (13), use of fluorescence markers would also aid the integration of robotic navigation with bimodal tracers (14).
In this study, we exploit real-time fluorescence-based optical tracking for navigation of a drop-in γ-probe during robotic procedures on phantoms and a porcine model. To increase the translational potential of this concept, we used image-guided surgery technologies already available in the clinic (Drop-In [Eurorad S.A.] (2), multicolor fluorescence (13), and declipseSPECT [SurgicEye GmbH]).
MATERIALS AND METHODS
Tracking and Navigation Setup
The SPECT-navigated setup (Fig. 1) consisted of the Drop-In γ-probe (3), a Da Vinci Si robot (Intuitive Inc.) with a Firefly fluorescence laparoscope (Intuitive Inc.), and a declipseSPECT navigation system (SurgicEye GmbH) with near-infrared optical tracking (Northern Digital Inc.), running customized tracking software. The Firefly Si laparoscope records the raw fluorescence video feed and allows for simultaneous visualization of fluorescein in yellow, indocyanine green (ICG) in pink, and the anatomic background in blue or in black and white (13). To allow for accurate calculations within the tracking software, the Firefly intrinsic and extrinsic camera properties were calibrated (15).
Overview of Drop-In tracking-and-navigation setup displaying underlying pose relations with colored arrows, describing the relative positions and orientations of all objects in the navigation workflow. (A) Near-infrared optical tracking determines the pose of Firefly and patient reference targets (red arrows), whereas underlying registrations translate this pose to Firefly camera (purple arrow) and lesions as found on SPECT/CT (orange arrow). (B) Vision-based tracking from Firefly determines the pose of Drop-In markings within surgical field (blue arrow), and these markings are calibrated with Drop-In probe tip (purple arrow). Finally, the Drop-In can be navigated toward SPECT/CT-marked lesions. NAV = navigation system; RT = reference target; T = pose transformation.
An asymmetric 3-ring fluorescent-marker pattern was incorporated into the Drop-In housing, fabricated from medical-grade ultraviolet-curable adhesives comprising fluorescein. The fluorescent emissions were automatically detected on the basis of color and shape. Using the known geometric arrangement, the pose of the Drop-In tip could be estimated (5 degrees of freedom) with respect to the Firefly laparoscope (Fig. 1B). With one reference target attached to the Firefly and one to the patient (Fig. 1A), the declipseSPECT was used to determine the pose of the Firefly and the patient within the operating room.
Phantom Evaluation
To study the concept of SPECT/CT-based navigation, we used a laparoscopic torso phantom (15) that contained bones, artery structures, and a radioactive model of pelvic lymph nodes (2 MBq of 99mTc each). Radioactive nodes were placed at different locations (i.e., right and left external iliac artery and left common iliac artery), and 3 separate SPECT/CT scans were acquired with the navigation reference target fixated at the phantom’s hip, allowing for registration of the scans in the operating room.
Porcine Evaluation
To investigate the Drop-In tracking setup in a real-life surgical setting, 4 pigs underwent robot-assisted laparoscopic surgery. Surgical targets were created by depositing ICG (∼50 μL, 2.5 mg/mL solution in saline) in the abdominal wall (average of 3 depositions per pig). The tracer deposits were manually set as targets to which the Drop-In probe was navigated. Animal experiments were performed under the approval of the local ethical committee.
RESULTS
Phantom Evaluation
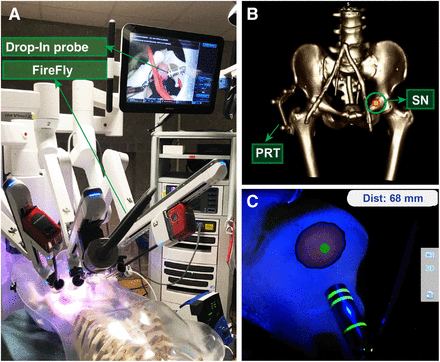
Figure 2 illustrates the concept of performing SPECT-based navigation of the Drop-In in the robot-assisted laparoscopic setting (see also Supplemental Video 1; supplemental materials are available at http://jnm.snmjournals.org). A navigation-enriched view of the abdomen was visible in the robotic console, including an augmented-reality overlay of the lesion segmented from SPECT/CT. Maintaining a direct line-of-sight between the Firefly and the Drop-In allowed for real-time calculation of the distance between the targeted lesion and the Drop-In tip in millimeters. The marker geometry allowed for a great range of maneuverability: at a 10-cm distance in line with the laparoscope, Drop-In tracking was feasible for 0°–360° (roll), 0°–360° (pitch), and 15°–165° and 195°–345° (yaw). The location of the lesion targets (i.e., left and right external iliac artery and left common iliac artery) meant that the high maneuverability of the Drop-In was instrumental for intraoperative detection (2). The audible feedback provided by the Drop-In γ-probe confirmed effective navigation.
Real-time Drop-In tracking and navigation during robot-assisted laparoscopic surgery on phantom. (A) Setup overview. (B) Phantom SPECT/CT example, displaying patient reference target (PRT) and targeted lymph node (SN). (C) Augmented-reality visualization in surgical console, displaying Drop-In navigation toward lymph node (including calculated distance).
Porcine Evaluation
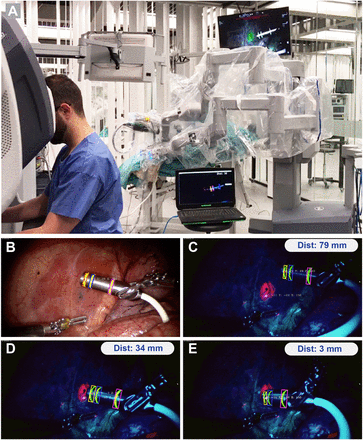
Robotic surgery on a porcine model was used to evaluate translation into an actual surgical setting (Fig. 3; Supplemental Video 1). In this real surgical environment, the fluorescein markers on the Drop-In probe remained clearly detectable (in yellow), both in white-light and fluorescence imaging mode. ICG-containing lesions were visible only in fluorescence imaging mode (in pink) and could be detected at the same time as the Drop-In markers (Figs. 3C–E). Uniquely, the fluorescent emissions of fluorescein (maximal λ-emission, 515 nm) and ICG (maximal λ-emission, 820 nm) could be excited simultaneously and could be distinguished by exploring the fluorescence-multiplexing capabilities of the Firefly Si camera (13). The surgeon was thus also able to visually confirm the localized lesions during navigation. Although the excitation power in the near-infrared region with this Firefly is roughly 100 times higher than in the blue region of the spectrum (13), there was no clear difference in intensity observed between the fluorescein markers and ICG lesions. Bleaching of the fluorescein ring markers was not observed within the approximately 1-h experiments. In addition, problems with blood contamination of the Drop-In markers were not observed, indicating the potential of fluorescence-based markers as discussed previously (12).
Real-time tracking of Drop-In probe with respect to targeted lesions during robot-assisted laparoscopic surgery on pig. (A) Operating room overview. (B) White-light image. (C–E) Tracking of Drop-In probe with respect to targeted lesion (pink) using fluorescence imaging at calculated target distances of 79, 34, and 3 mm.
DISCUSSION
We investigated the first steps toward optical navigation of the Drop-In γ-probe, a concept that further integrates interventional nuclear medicine during robotic surgery. Visualizing the position of the Drop-In with respect to predefined lesions is likely to help surgeons reduce intraoperative uncertainties about the target location and the γ-probe readout. The translational character of the findings is underscored by the proven utility of the Drop-In concept in prostate-cancer surgery (sentinel node targeting with both the tracer ICG-99mTc-nanocolloid and the tracer 99mTc-nanocolloid (2) and prostate-specific membrane antigen targeting with 99mTc-PSMA-I&S (5)), the use of medical-grade fluorescent materials as markers, the use of the clinically approved Firefly camera and declipseSPECT navigation system, and a proof of concept in porcine surgery. Obviously, the concept could in the future be disseminated to alternative indications for which 125I-, 99mTc-, or 111In-based radioguided surgery approaches have been reported (1) or even to alternative multicolor fluorescence approaches (16).
In this study, we evaluated navigation in preoperative SPECT/CT scans, but the same concept could also be expanded using, for example, a drop-in β-probe (or even a drop-in ultrasound probe) in combination with PET/CT scans (4). Unfortunately, the surgical facilities that we used to test the presented technologies did not allow us to evaluate SPECT/CT-based navigation in porcine models. Since to date a facility allowing such an experiment is unavailable, this link has to be further validated in clinical trials, as was done for other γ-probes (7). The current study, however, ensures that follow-up trials can rely on a validated technology, thus limiting the exposure of patients to unnecessary risks.
CONCLUSION
Surgical navigation of the Drop-In probe based on SPECT/CT is promising, providing the next step toward precision radioguided surgery, connecting nuclear medicine with robotic surgery. Studies on humans are in preparation to confirm these findings and evaluate the oncologic benefit of such an image-guided surgery approach.
DISCLOSURE
Financial support was received through NWO-TTW-VICI grant 16141, and hardware support was received from Eurorad S.A. Matthias van Oosterom, Fijs van Leeuwen, and Krijn Houwing have a pending patent on fluorescent-marker tracking. During this research, Elio Mazzone, Kevin Bauwens, Paolo Dell’Oglio, and Fijs van Leeuwen were partially affiliated with Orsi Academy. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is it possible to navigate the Drop-In γ-probe toward preoperatively marked lesions during robotic surgery?
PERTINENT FINDINGS: As evaluated in a robot-assisted phantom setting, fluorescence markers can be used to navigate the Drop-In toward lesions as identified on SPECT/CT. The feasibility of this concept was confirmed during surgery in a porcine model.
IMPLICATIONS FOR PATIENT CARE: Proving its utility in large-animal models, a next step has been taken toward precision radioguided surgery in the human robot-assisted setting.
Acknowledgments
We acknowledge Danny van Willigen, Tessa Buckle (LUMC, The Netherlands), and the Orsi Academy staff (Belgium) for assistance during surgical evaluations, as well as the Skills-Lab and Petra Dibbets (LUMC) for assistance during phantom evaluations.
Footnotes
Published online January 8, 2021.
- © 2021 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication November 5, 2020.
- Accepted for publication January 3, 2021.