Abstract
Peptide receptor radionuclide therapy (PRRT) with 177Lu-labeled somatostatin analogs in patients with somatostatin receptor–expressing tumors is often performed using administration protocols prescribing a 30-min infusion time. The most often used method of infusion is the gravity method, by which the complete dose is effectively administered exponentially. However, there is no evidence to explicitly support an infusion time of 30 min. This study aims to investigate the safety of an infusion time of less than 5 min. Methods: A cohort study was performed, examining the biochemical and clinical toxicity after PRRT when using a fast-infusion protocol with a maximum infusion time of 5 min. Data on patient characteristics, laboratory tests, follow-up visits, and pre- and posttreatment imaging using 68Ga-DOTATOC PET/CT from patients treated with PRRT at the University Medical Center Utrecht (UMC Utrecht) were collected. All patients receiving PRRT using the fast-infusion protocol were included. If no laboratory or clinical follow-up was available, patients were excluded. In addition, a laboratory experiment was performed, simulating the standard-infusion protocol using the gravity method. Results: Thirty-one patients, treated using the fast-infusion protocol, were included. Clinical toxicity mainly consisted of grade 1/2 fatigue (87.1%) and grade 1 nausea or vomiting (67.7%) during follow-up. No acute or long-term clinical toxicity possibly related to the fast-infusion protocol was reported. Grade 3/4 hematologic toxicity occurred after PRRT in 1 patient (3.2%). No grade 3/4 renal toxicity occurred. The laboratory experiment showed that when using the gravity method for infusion, half of the activity is infused after 3.5 min, and 95% is infused within 15 min. Conclusion: A faster infusion of PRRT using an infusion time of less than 5 min is safe and feasible in clinical practice.
Peptide receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE/DOTATOC/HA-DOTATATE is increasingly being used for treatment of inoperable grade I/II neuroendocrine neoplasms (NENs) (1–4). PRRT uses the radioactive isotope 177Lu, coupled to a peptide that mainly targets somatostatin subtype 2 receptors. These receptors are often highly overexpressed on the cell surface of NENs and some other type of tumors (5,6). The radiopharmaceutical binds to the receptor with high affinity and is internalized by the tumor cells after intravenous administration. The presence of the somatostatin subtype 2 receptors can easily be made visible by 68Ga-somatostatin receptor PET/CT (68Ga-SSTR PET/CT) (7). The treatment is safe, as toxicity is limited to grade I–II hematotoxicity in less than 10% of patients and is often transient (3,8,9). Grade III or IV hematotoxicity or a decreased renal function rarely occur after treatment with PRRT. When PRRT is administered, multiple precautionary measures are taken to ensure a safe infusion for the patient as well as the health-care personnel performing the administration (10,11). According to the instructions from the manufacturer of 177Lu-DOTATATE (Lutathera; Advanced Accelerator Applications, Novartis), the prescribed method of administration is the so-called gravity method. This method uses gravity to flush the vial containing the radiopharmaceutical with saline solution for injection (0.9 mg/mL), while regulating the flow to a specific infusion rate. The instructed duration of the infusion is 30 min, during which a constant flow of 400 mL/h should be maintained. The infusion time was set to be 30 min when the first results on patients treated with PRRT were published, and all subsequently published protocols adapted this 30-min infusion time (1,12). However, other administration methods are considered, often using a pump to ensure a more constant infusion in PRRT (13,14). By using a pump, a faster infusion time can be achieved. However, there are concerns when infusing at a faster rate, due to the lack of safety studies. In the current study, we investigate the safety of a faster infusion in PRRT by analyzing toxicity profiles after PRRT using the fast-infusion protocol, in which a total intravenous infusion time of 5 min is used.
MATERIALS AND METHODS
Patients
All patients treated with PRRT (in-house–labeled 177Lu-HA-DOTATATE; Scintomics) using the fast-infusion protocol (regular care in our hospital since March 2017) from September 2016 until April 2019 were included. 177Lu-HA-DOTATATE was prepared in house using a semiautomated Modular-Lab eazy synthesis module (Eckert & Ziegler). Each synthesis was performed according to the manufacturer’s instructions using a prefabricated cassette, a good-manufacturing-practices-grade ascorbic acid buffer, a C18 cartridge, and a 0.22-μm pore size sterilization filter. An amount of 50 μg of HA-DOTATATE (Scintomics) per GBq of 177Lu (EndolucinBeta; ITM Medical Isotopes GmbH) was used. The pH of the batches of 177Lu-HA-DOTATATE was 4.7 (range, 4.2–5.2). Per patient, the batch was diluted with 0.9% NaCl to 8 mL for infusion of 7.4 GBq of 177Lu-HA-DOTATATE with an osmolality of 272–287 mOsm/kg.
Treatment indications for all patients were discussed in a multidisciplinary tumor board. The European Association of Nuclear Medicine guidelines were followed to include patients for treatment (15). All patients had sufficient SSTR expression on 68Ga-DOTATOC/HA-DOTATATE PET/CT (i.e., more than healthy liver tissue) and had an inoperable NEN or other type of tumor for which there was no other treatment option. Patients who received at least 1 cycle of PRRT were included. Patients were excluded if laboratory investigations on hepatic, hematologic, and renal function before or after treatment were not available. As this is a retrospective study, the need for approval of the study protocol and informed consent by the included patients were waived.
Study Procedures
Patients were screened by the nuclear medicine physician. Complaints and physical examination were recorded at baseline, and laboratory investigations were performed (i.e., renal function, hepatic function and enzymes, and hematologic status). Baseline toxicity was recorded. Patients were admitted to the radiation ward on the day of administration. Preparation consisted of new laboratory investigations, a single dose of 8 mg of ondansetron intravenously 30 min before administration, and coinfusion of an amino-acid solution (1 L of arginine/lysine 2.5%/2.5%). After treatment, patients were discharged from the hospital according to local radiation safety regulations. A control visit was planned 4–6 wk after PRRT. If patients received more than 1 treatment cycle, treatment intervals were in between 6 and 9 wk.
Administration Method
A commercially available shielded administration pump (RAD-INJECT; Tema Sinergie) was used to provide a constant flow during infusion, with a fixed volume of 12 mL. A shielded syringe with the radiopharmaceutical was loaded into the pump and connected to a side port of the infusion system used for amino-acid coinfusion. Directly after infusion, the pump extracted a fixed volume from a bag of regular saline solution and flushed the entire system twice to administer any possible remnant of the radiopharmaceutical. A fast-infusion time of 5 min was used (including flushing of the system twice; actual radiopharmaceutical infusion in 1.5 min). During infusion, patients were closely monitored for any complaints or adverse effects.
Outcomes
Toxicity
The primary outcome of this study was the occurrence of adverse events during or after administration of PRRT. Clinical and laboratory-related adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 ( 16 ). Adverse events were reported only if the grade was higher than at baseline. Clinical toxicity was recorded at the day of each treatment cycle and during each follow-up visit 4–6 wk after each treatment cycle. Laboratory investigations were obtained during follow-up visits after each treatment cycle and regularly after completion of PRRT up until 1 y after the first cycle.
Response
As a secondary outcome, objective response was evaluated after treatment using 68Ga-DOTATOC PET/CT imaging. Response was evaluated only in included patients for whom 68Ga-DOTATOC PET/CT imaging was available at baseline and after PRRT. Volumes of interest (VOIs) were drawn semiautomatically using Syngo.Via (Siemens), based on the recommendations adapted from the PERCIST guidelines ( 17 ). Regions with high uptake of 68Ga-DOTATOC were segmented automatically using a threshold based on a spheric VOI, which was placed in the healthy liver tissue. Tissue with physiologic SSTR expression was manually removed from the resulting delineation (i.e., kidneys, bladder, spleen, pituitary gland, adrenals, and small intestine). On the basis of the final VOIs, the SUVpeak (corrected for lean body mass), total lesion SSTR expression (TL-SSTR, derived from total lesion glucolysis), and SSTR-expressing tumor volume (SSTR-TV, derived from metabolic tumor volume) were calculated. SUVpeak, TL-SSTR, and SSTR-TV calculated from the baseline and follow-up PET/CT were compared, to assess the response in patients. According to PERCIST guidelines, a threshold of 30% decrease or increase in SUVpeak was used to categorize patients as demonstrating partial metabolic response, stable metabolic disease, or progressive metabolic disease.
Laboratory Experiment
To assess the flow of the radiopharmaceutical with the gravity method, the setup for administration was simulated in our nuclear laboratory at UMC Utrecht (Fig. 1). In 4 subsequent infusion simulation experiments, a glass vial sealed with a rubber septum was filled with 99mTc-pertechnetate (99mTcO4
−) in variable amounts of saline solution (0.9% sodium chloride in water). A normal saline 0.9% sodium chloride 250-mL bag for injection was connected to the vial via a regular infusion line and a short 19-gauge needle that was placed through the septum of the vial (the afferent system). A long 20-gauge needle was also placed through the septum with the tip of the needle at the bottom of the vial and connected to an infusion line, leading toward a waste container for radioactive material (the efferent system). An infusion pump was connected to the efferent line to accurately control the flow. The pump was set to pump 200 mL at a rate of 400 mL/h, resulting in an infusion time of exactly 30 min. The vial containing the 99mTcO4
− solution was placed in a dose calibrator, after which injection was initiated. The amount of radioactivity was denoted every 60 s, starting at 0 min until 30 min. The measured activity was corrected for background radiation and physical decay. The activity was plotted as a fraction of the total activity against the infusion time. Because this setup essentially is a continuous dilution of the contents of the vial, an exponential depletion curve was fitted to the data using nonlinear regression and compared with the observed data. The formula used for exponential depletion was , where
, where  was the activity fraction in the vial at the start of the infusion (i.e., 100%), λ was the decay constant, and
was the activity fraction in the vial at the start of the infusion (i.e., 100%), λ was the decay constant, and  was the time since start of infusion in minutes.
was the time since start of infusion in minutes.
Schematic of the laboratory experiment setup. (A) Bag of 0.9% saline solution. (B) Dose calibrator. (C) Vial with dissolved 99mTc, into which a long and a short needle are inserted. (D) Regular infusion pump. (E) Radioactive waste container.
Statistical Analysis
Trends in laboratory findings (i.e., bilirubin, creatinine, thrombocytes, and leukocytes) were examined using linear mixed models. To model the correlation of longitudinal data, an autoregressive correlation model was used. Furthermore, a random intercept and random effect of time on laboratory results were implemented. The models were checked on normality of the residuals. All statistical analysis was done using R (R Core Team 2020, version 3.6.2). P values of less than 0.05 were considered statistically significant.
RESULTS
Clinical Toxicity
A total of 31 patients were included, who received a total of 99 PRRT cycles (Table 1) . Patients did not report any acute toxicities during or directly after administration. Patients reported multiple CTCAE adverse events, ranging from grade 1 to grade 3 (Table 2). Most patients complained of grade 1 or 2 fatigue, which frequently occurred in the weeks after the administration (27/31 [87.1%]). Other commonly reported adverse reactions were grade 1 or 2 nausea or vomiting (21/31 [67.7%]). Two grade 3 adverse events occurred in 2 patients. The first patient suffered from extreme fatigue after receiving 1 cycle of PRRT. The fatigue caused further treatment with PRRT to be postponed and eventually be canceled due to worsening of the clinical condition of the patient. Laboratory findings did not reveal a specific cause for the fatigue. The second patient had preexisting major carcinoid-related complaints (flushing and diarrhea, both > 10 times a day) and suffered from a carcinoid crisis hours after the first treatment cycle. One day after administration, the patient reported increasing complaints of flushing, diarrhea, cardiac arrhythmias, and dyspnea, despite being adequately treated with short-acting octreotide. Being hemodynamically stable with a normal blood pressure and heart rate, the patient was hospitalized and received a bolus dose of 500 μg of octreotide, as well as continuous intravenous octreotide at 50 μg/h. Over the course of a couple of days, the symptoms reduced and octreotide infusion was stopped. During subsequent PRRT administrations, additional care was taken to prevent the occurrence of a carcinoid crisis by preemptive intravenous administration of octreotide 24 h after PRRT. The patient completed all 4 treatment cycles of PRRT.
Baseline Characteristics
Clinical Toxicity
Clear trends of decreasing thrombocyte, leukocyte, and hemoglobin levels were found after PRRT (Fig. 2; P < 0.001, P < 0.001, and P = 0.002, respectively), however, no significant change over time in creatinine levels was observed (P = 0.267). In terms of CTCAE grading, grade 1 or 2 hematologic toxicity occurred in 21 (67.7%) of patients (Table 3). Grade 3 hematologic toxicity occurred only once in a patient with thrombocytopenia, which resolved completely after a few months. Grade 1 or 2 renal toxicity occurred in 4 (12.9%) patients treated using the fast-infusion protocol. No grade 4 or 5 toxicity was observed in the study period.
Several laboratory trends in the 50 wk after first cycle of PRRT. Trends indicate median level among patients in indicated time period. Error bars indicate interquartile range.
Biochemical Toxicity
Objective Response
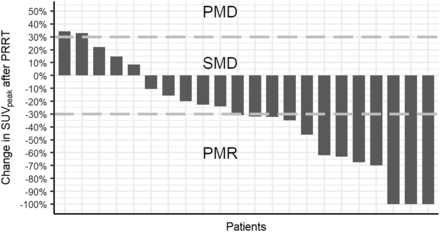
Objective response using 68Ga-DOTATOC PET/CT imaging was measured in 22 patients, for whom imaging was available at baseline and after PRRT (Table 1). In 9 patients, 68Ga-DOTATOC PET/CT imaging was not available after treatment. On the basis of on SUVpeak values, partial metabolic response was achieved in 12 (54.5%), stable metabolic disease in 8 (36.4%), and progressive metabolic disease in 2 (9.1%) patients. Median decrease in SUVpeak was 31.4% (interquartile range [IQR], 11.7%–62.8%), whereas median decrease in TL-SSTR and SSTR-TV was 66.5% (IQR, 42.2%–82.8%) and 66.7% (IQR, 28.8%–79.3%), respectively (Fig. 3). In 3 patients in whom a complete reduction in SUVpeak was calculated, there was still viable tumor visible on 68Ga-DOTATOC PET/CT, which, however, was too little to be included in the automatically delineated VOIs. Therefore, these patients were classified as partial response, rather than complete response (Fig. 3).
Response by SUVpeak on 68Ga-DOTATOC PET/CT after PRRT. Value at post-PRRT indicates best response if multiple scans after PRRT were analyzed.
Laboratory Experiment
A total of 4 vials that contained 709, 398.9, 289, and 320.8 MBq of 99mTc in 23, 20, 23, and 21.5 mL of saline solution were flushed using the gravity method, respectively (Fig. 4). In vial 1, the observed line deviated from the supposed ideal decay curve at approximately 8 min. This was explained by leakage of air from the vial through the septum, causing the vial to contain more fluid after a certain period. In all other vials, the dilution process followed the exponential decay curve. The median estimated decay constant was 0.2477 (range, 0.1891–0.3224). The median time until 50% of the radioactivity was infused was 3.5 min (range, 3–4 min), for 75% 6.5 min (range, 5–8 min), and for 95% 15 min (range, 10–29 min).
Flow of 99mTc by flushing (gravity) method. Measured fraction of activity remaining in vial at time since start of infusion per vial (dots) and their corresponding fitted decay curves (dashed lines). Note deviation from fitted curve in vial 1 at approximately 8 min of infusion time.
DISCUSSION
In the current study, we have shown that it is safe to administer PRRT in a time span of at least 5 min (this includes flushing the administration system twice, thus infusing the radiopharmaceutical in only 1.5 min). No additional toxicity was observed, and response rates according to 68Ga-DOTATOC PET/CT scans were good. The added benefit of using the gravity method for administering PRRT is questionable, as the laboratory experiment showed that with the gravity method 95% of the dose is administered in 15 min, and 50% in 3.5 min.
The current study shows that a total infusion time of 5 min (including thorough flushing of the system) with a constant infusion rate is safe. In terms of clinical and biochemical toxicity, no additional occurrence of adverse events was found. The toxicity profiles in this study are similar to previous reported studies. Brabander et al. published a cohort of 582 patients, in which grade 3 or 4 combined hematologic toxicity (thrombocytopenia, leukopenia, and anemia) was observed in 10% of patients ( 18 ). In the NETTER-1 trial, grade 3/4 thrombocytopenia occurred in 2% of patients, grade 3/4 leukopenia occurred in 1% of patients, and grade 3/4 anemia was not seen after PRRT. In the current study, overall hematologic toxicity was quite similar (3.2% of patients, due to thrombocytopenia). There was no grade 3/4 anemia or leukopenia in the current study population. However, Brabander et al. reported grade 3/4 lymphocytopenia after PRRT in 50% of patients, whereas the NETTER-1 trial reported the same in 9% of patients. In the current study, lymphocyte counts were not analyzed, as it was not measured regularly in all patients. As observed in both mentioned studies by Brabander et al. and the NETTER-1 investigators, renal toxicity after PRRT is rare, most importantly due to the combined infusion of an amino-acid solution during treatment. Grade 3/4 renal toxicity is generally reported in 1% of treated patients, but in our cohort, no patients had grade 3 or 4 renal toxicity. Therefore, and considering the infusion curve of the 30-min protocol, a fast infusion of PRRT has probably no effect on renal toxicity. Furthermore, the occurrence of clinically reported adverse events was comparable to the rates reported in previously published studies, and no specific complaints were reported by any patient during the infusion of the radiopharmaceutical ( 3,18 ). The NETTER-1 study reported significantly increased nausea and vomiting after PRRT compared with standard SSA (somatostatin analog) treatment, with rates of 59% for nausea and 47% for vomiting. In the current study, nausea or vomiting occurred in 67.7% of patients (grade 1/2). The most frequently encountered complaint in this study was the occurrence of grade 1 or 2 fatigue, which is often preexistent, but worsening during treatment. Although (worsened) fatigue during PRRT is commonly described in different cohorts, in the long term a decrease in fatigue is often observed. In the NETTER-1 trial, the hazard for the time-to-deterioration of fatigue was significantly lower in patients treated with PRRT than in patients treated only with SSA ( 9 ). The relatively high occurrence of fatigue in this study can be explained by the high frequency of check-ups, during which every mention of fatigue is counted as an adverse event (i.e., before treatment, during each treatment cycle, and after each treatment cycle).
Response after PRRT is usually measured using contrast-enhanced CT or MRI ( 2 ). Because of the retrospective nature of this study, follow-up imaging using contrast-enhanced CT or MRI was not feasible. Therefore, comparison with literature may be difficult. However, in clinical practice, 68Ga-DOTATOC imaging is increasingly being used during follow-up, especially in low-grade NENs. In our study, response rates after PRRT based on 68Ga-DOTATOC PET/CT imaging were rather good, with a partial response rate of 54.5% and stable disease in 36.4% of patients. Progressive disease was seen in 9.1% of patients.
There are many suggested techniques for safe infusion in PRRT ( 19 ). The gravity method is the first and most widely implemented technique due to the adaptation after the first clinical trials. An advantage of this method is that no manipulation of the content of the vial is necessary, and only basic materials are necessary for the entire setup. On the other hand, there can be problems leading to incomplete infusion of the radiopharmaceutical, such as air leakage from the vial due to incorrect placement of the needles or defects in the vial’s septum. This occurred in 1 of 4 of our attempts of the laboratory experiment, resulting in a slower infusion of the radiopharmaceutical and potentially higher residual activity. Therefore, the time until complete infusion may vary, even with the same amount of saline solution and configured infusion rate. Additionally, there is risk of contamination due to leakage of radioactivity from the vial ( 15 ). Apart from these disadvantages, the infused concentration of the radiopharmaceutical is not constant due to the flushing with regular saline fluid, resulting in infusion of most of the activity within the first minutes after initiation of infusion as shown in the laboratory experiment. Therefore, administration using the flushing (gravity) method essentially equates to infusing most of the initial dose in 5 min.
A second technique requires the use of a syringe pump (or intravenous pump system), into which the radiopharmaceutical solution has been aspirated before treatment ( 11 ). A similar method was also used in this study. Main advantages of implementing this technique include that there is no risk of air aspiration, and in our case, flushing of the entire system is also automated, limiting radiation exposure to personnel. Furthermore, the infusion rate is constant. Disadvantages are that equipment should be shielded and that the radiopharmaceutical has to be handled by laboratory technicians to prefill the shielded syringe.
In theory, slow infusion of the radiopharmaceutical exposes the SSTRs on the tumor cells to the compound for a longer period of time, hypothetically increasing the uptake. As a result, shortening the infusion could negatively affect the efficacy of PRRT. However, a shorter infusion time will likewise result in a higher blood concentration, which in turn potentially increases the saturation of SSTRs ( 7 ). Internalization of these highly saturated receptors would then result in a higher absorbed dose in the tumor cells. Longer infusion of the radiopharmaceutical is only useful to bind to recycled SSTRs, which is a process that is shown to complete only after 24 h ( 20,21 ). Hence, using the recycled SSTRs for treatment would require much longer infusion times. This is undesirable, as a longer infusion time presents added risks in terms of leakage, extravasation, and radiation exposure and is increasingly uncomfortable to the patient. In addition, the 177Lu decays to some extent. The exact influence of the administration method of somatostatin analogs on the binding and internalization process is still unclear. There are suggestions that a higher concentration of somatostatin analog within a short period of time results in a higher tumor-absorbed dose. This is shown by Braat et al. in a patient with meningioma receiving 177Lu-HA-DOTATATE both intravenously and intraarterially ( 22 ). Intraarterial infusion in the feeding artery of the tumor resulted in a high local blood concentration of the radiopharmaceutical. When both administration methods were compared, an 11-fold increase in tumor uptake was quantified on the posttreatment 177Lu planar scintigraphy after intraarterial administration. Another reason to shorten the infusion time is the fact that the agonist-induced desensitization and internalization process is quick and happens within minutes. Binding of the somatostatin analog is arguably the most significant within these first minutes.
Faster infusion may also affect the workflow of clinical personnel positively. During infusion, careful monitoring of the process is warranted, thus limiting the amount of patients that can be treated simultaneously, depending on the local procedural guidelines. With a shorter infusion time, a more efficient workflow may be possible.
In our study, there are some limitations. First, long-term clinical toxicity was not investigated. However, after fast infusion of PRRT, mainly short-term adverse reactions are of concern. Long-term negative effects of a fast infusion in PRRT are not expected. Second, lymphocyte count was not investigated, as data were not sufficiently available. In previous studies, a temporary lymphopenia occurred frequently. Third, response after PRRT was measured using 68Ga-DOTATOC PET/CT. Even though this imaging technique is a promising tool in the follow-up of PRRT-treated patients, no evidence exists on its validity. In 9 patients, PET/CT scans were not available for response assessment. In 8 patients, follow-up consisted of conventional imaging (i.e., contrast-enhanced CT/MRI), and in 1 patient the image quality of the PET/CT scan was insufficient to analyze the response. Fourth, the study population is heterogeneous, as any type of tumor was included. However, as the aim of the study was toxicity after fast infusion of PRRT, this does not affect the validity of the study. Last, specific characteristics of different radiopharmaceuticals, such as pH, could in theory affect complaints observed by the patient. In this study, these factors could not be considered because only 1 radiopharmaceutical was used.
Because our study is a cohort study, no direct comparison can be made with PRRT using the standard 30-min protocol. In the future, a randomized controlled trial could be performed comparing both infusion protocols to establish final certainty on the most adequate method of infusion in PRRT. Both the effect on toxicity and on response should be investigated, as the effect of fast infusion on the saturation of SSTRs is still unclear.
CONCLUSION
Rapid administration of PRRT in 5 min is feasible and can be safely used in standard clinical practice.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is it safe to administer PRRT with an infusion time of 5 min?
PERTINENT FINDINGS: In a cohort study in which patients with NET were treated with PRRT using a fast-infusion protocol, no related clinical or biochemical toxicity was found. Unrelated toxicity profiles are similar to those found in regular PRRT infusion, with grade 1/2 fatigue (87.1%) and grade 1 nausea or vomiting (67.7%) being the most frequent, and grade 3/4 hematologic toxicity occurring in 1 patient (3.2%).
IMPLICATIONS FOR PATIENT CARE: A faster infusion of PRRT is less time-consuming for patients and health-care professionals, with no added risk compared with other infusion protocols.
Footnotes
Published online Nov. 27, 2020.
- © 2021 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication July 6, 2020.
- Accepted for publication September 29, 2020.