Abstract
Over 20 different prostate-specific membrane antigen (PSMA)–targeting radiopharmaceuticals for both imaging and therapy have been synthesized. Although variability in biodistribution and affinity for binding to the PSMA receptor is known to exist between different PSMA-targeting radiopharmaceuticals, little is known about the clinical implications of this variability. Therefore, this study analyzed differences in interreader agreement and detection rate between 2 regularly used 18F-labeled PSMA receptor–targeting radiopharmaceuticals: 18F-DCFPyL and 18F-PSMA-1007. Methods: One hundred twenty consecutive patients scanned with 18F-PSMA-1007 were match-paired with 120 patients scanned with 18F-DCFPyL. All 240 PET/CT scans were reviewed by 2 readers and scored according to the criteria of the PSMA Reporting and Data System. Interreader agreement and the detection rate for suspected lesions were scored for different anatomic locations such as the prostate, prostatic fossa, lymph nodes, and bone. Results: Great equality was found between 18F-DCFPyL and 18F-PSMA-1007; however, some clinically relevant and statistically significant differences were observed. 18F-PSMA-1007 detected suspected prostatic or prostatic fossa lesions in a higher proportion of patients and especially in the subcohort scanned for biochemical recurrence. 18F-DCFPyL and 18F-PSMA-1007 showed an equal ability to detect suspected lymph nodes, although interreader agreement for 18F-DCFPyL was higher. 18F-DCFPyL showed fewer equivocal skeletal lesions and higher interreader agreement on skeletal lesions. Most of the equivocal lesions found with 18F-PSMA-1007 at least were determined to be of nonmetastatic origin. Conclusion: Clinically relevant differences, which may account for diagnostic dilemmas, were observed between 18F-DCFPyL and 18F-PSMA-1007. Those findings encourage further studies, as they may have consequences for selection of the proper PSMA-targeting radiopharmaceutical.
In recent years, prostate-specific membrane antigen (PSMA) receptor PET/CT has rapidly evolved as a cornerstone in prostate cancer imaging. PSMA PET/CT outperforms other imaging modalities because of its superior sensitivity and specificity, although sensitivity for lymph node metastases has been found to be moderate in prospective trials (1–5). The better diagnostic characteristics account for better treatment selection in both primary staging and biochemical recurrence. The ability to detect small metastases, for example, may benefit patients with oligometastatic disease, offering them treatment options with a chance of cure or survival benefits (6,7), although scientific underpinning of the latter is needed (8). The additional value of PSMA PET/CT during follow-up of systemic treatment, including androgen deprivation therapy and chemotherapy, in the palliative phase of the disease also needs to be clarified.
Because of the success of PSMA PET/CT, the number of different radiopharmaceuticals targeting the PSMA receptor has increased significantly (9). Although most early publications on the clinical use of PSMA PET/CT reported on findings with 68Ga-labeled radiopharmaceuticals, later publication also gave attention to 18F-labeled radiopharmaceuticals. Positrons emitted by 18F decay have lower kinetic energy than those emitted by 68Ga, resulting in a higher resolution in PET images acquired using 18F tracers. Furthermore, the 110-min half-life of 18F, compared with 68 min for 68Ga, enables imaging at later time points without significant deterioration of image quality or the need to administer higher doses. This point is of clinical importance since PSMA tracer kinetics show that the tracer accumulates in prostate cancer cells over time whereas the background activity decreases (10–14). Although over 20 different PSMA-targeting radiopharmaceuticals for both imaging and therapy have been synthesized, only a few are used in common clinical practice.
Variability in biodistribution and affinity for binding to the PSMA receptor is known to exist between the different PSMA-targeting radiopharmaceuticals, but little is known about the clinical implications of this variability (15,16). This lack is reflected in recent EAU guidelines that do not discriminate between different PSMA-targeted radiopharmaceuticals for imaging of prostate cancer (https://uroweb.org/guideline/prostate-cancer/). To investigate whether these differences are clinically important and interfere with the reproducibility of scan outcomes and lesion detection, this study analyzed—in a large matched-pair cohort of 240 patients—interreader agreement and detection rate for suspected lesions using 2 common 18F-labeled PSMA receptor–targeting radiopharmaceuticals: 2-(3-(1-carboxy-5-[(6-18F-fluoro-pyridine-3-carbonyl)-amino]-pentyl)-ureido)-pentanedioic acid (18F-DCFPyL) and (3S,10S,14S)-1-(4-(((S)-4-carboxy-2-((S)-4-carboxy-2-(6-18F-fluoronicotinamido)butanamido)butanamido)methyl)phenyl)-3-(naphthalen-2-ylmethyl)-1,4,12-trioxo-2,5,11,13-tetraazahexadecane-10,14,16-tricarboxylic acid (18F-PSMA-1007). Known differences between these 2 radiopharmaceuticals that may interfere with scan readability include differences in excretion pathways and bone marrow uptake (15,16). Renal excretion of 18F-DCFPyL results in high activity in the urinary tract, which may interfere with detection of lesions near the ureters and urinary bladder, whereas activity in the urinary tract is usually less for 18F-PSMA-1007 because biliary excretion is the most important excretion pathway. Physiologic bone marrow uptake is commonly higher for 18F-PSMA-1007 and may interfere with detection of bone metastases.
MATERIALS AND METHODS
Patient Population
This study retrospectively included 120 consecutive patients imaged between April 2, 2019, and June 20, 2019, with 18F-PSMA-1007 PET/CT for primary staging, biochemical recurrence, or follow-up of systemic treatment of prostate cancer. In addition, 120 patients who were imaged between November 3, 2016, and March 21, 2019, with 18F-DCFPyL were extracted from a prospectively maintained database of 813 patients scanned with 18F-DCFPyL. To allow for a fair comparison of these 2 cohorts, they were matched on the basis of disease stage (primary staging, biochemical recurrence, or follow-up of treatment for castration-resistant prostate cancer); prostate-specific antigen (PSA) level at the time of PET/CT (PSA difference of <10% between 2 patients); and, in cases of biochemical recurrence, previous treatment (prostatectomy, lymph node dissection, external radiation therapy, or brachytherapy).
Besides the standard PSMA PET/CT acquired for clinical indications, no additional measurements or actions affecting the patient were performed. The study was approved by the local scientific board, and the need to receive approval from the local ethical committee was waived because the study did not fall within the scope of the Dutch Medical Research Involving Human Subjects Act (section 1.b; February 26, 1998). Additionally, as a standard procedure in our department, all included patients gave written consent to use of their anonymized data for scientific purposes.
Image Acquisition
18F-DCFPyL and 18F-PSMA-1007 were synthesized by an on-site cyclotron facility. PET images were acquired on a Biograph-16 TruePoint PET/CT scanner (Siemens Healthcare) at 120 min after injection of 18F-DCFPyL (mean of 319 MBq and range of 231–367 MBq, depending on body mass) or 90 min after injection of 18F-PSMA-1007 (mean of 324 MBq and range of 239–363 MBq, depending on body mass). Images were acquired from the inguinal region to the base of the skull (5 min per bed position). Data were reconstructed using an iterative ordered-subset expectation maximization 3-dimensional algorithm using 4 iterations, 16 subsets, and a 5-mm gaussian filter. The image matrix size was 256 × 256, pixel spacing was 2.67 × 2.67 mm, and slice thickness was 4 mm. For attenuation correction, a radiocontrast-enhanced CT scan (110 mAs at 110–130 kV) was typically acquired. Collimation was 16 × 1.2 mm; pitch, 0.95; slice thickness, 4 mm; and matrix size, 512 × 512. The resulting voxel sizes were 1.37 × 1.37 mm for CT images for attenuation correction and 0.98 × 0.98 mm for diagnostic CT images.
Data Acquisition
All 240 PET/CT scans were reevaluated by 2 nuclear medicine physicians with ample experience in reading both 18F-DCFPyL and 18F-PSMA-1007 PET/CT (each reader had >300 readings of both 18F-DCFPyL and 18F-PSMA-1007). The readers had access to limited patient data, including clinical indication for PSMA PET/CT, PSA level at time of PSMA PET/CT, Gleason score, TNM stage if known before PSMA PET/CT, and previous treatments. Readers were masked to all other data, including the used radiopharmaceutical.
The scan outcome was scored for different anatomic localizations, including prostate or prostatic fossa, inguinal lymph nodes, pelvic lymph nodes (N1 nodes, according to the TNM system in the eighth edition of the AJCC Cancer Staging System), abdominal lymph nodes, thoracic lymph nodes, axillary lymph nodes, cervical lymph nodes, pelvic bones, vertebral bones, thoracic bones (costae, sternum, claviculae, and scapulae), bones of the extremities, and other suspected lesions. The scans were read using the criteria of the PSMA Reporting and Data System (RADS) (17). For each anatomic localization, scan outcomes were entered in a database. Only the lesions with the highest score or highest likelihood of malignancy according to the reading system were recorded. If one or both readers found only equivocal lesions outside the prostate or prostatic bed, the true nature of the equivocal finding was retrospectively determined using data from histopathologic biopsies, imaging at follow-up, or PSA response to therapy. Definitive proof of nonmetastasized disease included, first, histopathologic findings excluding metastasized disease and, second, complete biochemical response after local therapies including prostatectomy, lymphadenectomy, or radiation therapy without or after discontinuation of androgen deprivation therapy and without local therapy of the equivocal findings. Definitive proof of metastasized disease included concordant findings on MRI, progression of lesions on follow-up PSMA PET/CT, or PSA response to targeted therapy that was administered because of equivocal findings.
Statistical Analysis
The Mann–Whitney U test was used to test for differences between 18F-DCFPyL and 18F-PSMA-1007 outcomes. Interreader agreement was measured by calculating the weighted Cohen κ and the percentage of agreement. As conventionally done, agreement was categorized as poor, fair, moderate, good, or very good, reflecting agreement of 0%–19%, 20%–39%, 40%–59%, 60%–79%, and 80%–100%, respectively. Weighted κ is highly dependent on the proportion of positive scan results; therefore, a low weighted κ may reflect good or even very good agreement when proportions of positivity are relatively low (?<0.2) or high (?>0.8) (18). For calculation of differences between 18F-DCFPyL and 18F-PSMA-1007 and agreement between readers, some subcategories of the PSMA RADS reading system were merged since some subdivisions barely have clinical impact: 1a, 1b, and 2 (benign and likely benign); 3a, 3b, 3c, and 3d (equivocal); and 4 and 5 (likely malignant and malignant). Pie plots were constructed for outcomes that showed relevant differences. All analyses were performed using the Statistical Package for Social Sciences (SPSS Statistics, version 25.0; IBM).
RESULTS
In total, 240 18F-PSMA PET/CT scans—120 18F-DCFPyL and 120 18F-PSMA-1007—were included in the study (Table 1). Commonly found differences between 18F-DCFPyL and 18F-PSMA-1007 uptake are shown in Figure 1.
Patient Characteristics
Distribution of 18F-DCFPyL and 18F-PSMA-1007 in patients imaged for primary screening of high-risk prostate cancer, all scored as negative for bone and lymph node metastases by 2 experienced readers. Maximum-intensity-projection images are scaled to SUV-body weight of 0.0–10.0. Lower physiologic 18F-DCFPyL uptake is observed in liver, spleen, and bone marrow. Lower 18F-DCFPyL uptake is observed in primary prostate tumor. Low 18F-PSMA-1007 activity is seen in urinary tract and trachea. 18F-PSMA-1007 uptake in skeleton is heterogeneous between patients; no uptake to diffuse uptake is observed in bone marrow, and variable patterns of irregular 18F-PSMA-1007 uptake are observed in ribs of several patients. Higher 18F-PSMA-1007 activity in bile ducts and gallbladder is not appreciated because of scaling.
Prostate and Prostatic Fossa
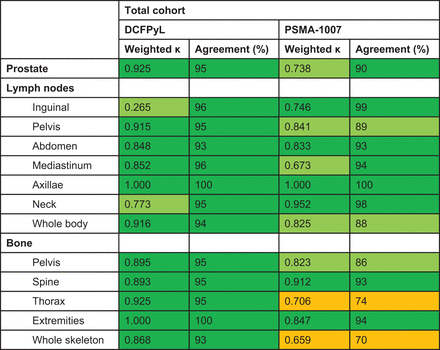
For the prostate and prostatic fossa, interreader agreement was very good for 18F-DCFPyL and good for 18F-PSMA-1007 (Fig. 2). Both readers found significantly more suspected prostate lesions with 18F-PSMA-1007 using the PSMA RADS criteria (Table 2; Supplemental Fig. 1; supplemental materials are available at http://jnm.snmjournals.org). Categorization of the cohort according to disease stage shows statistical differences for detection of suspected prostate lesions by both readers for patients with biochemical recurrence only (Table 3).
Interreader agreement reflected by weighted Cohen κ and by percentage for both 18F-DCFPyL and 18F-PSMA-1007. Dark green = very good agreement; light green = good agreement; orange = moderate agreement.
Detection Rates of Suspected Lesions and Equivocal Lesions and Testing of Equality for Detection of Suspected Lesions Between 18F-DCFPyL and 18F-PSMA-1007 for Total Cohort
Detection Rates of Suspected Lesions and Equivocal Lesions and Testing of Equality for Detection of Suspected Lesions Between 18F-DCFPyL and 18F-PSMA-1007 According to Primary Staging, Biochemical Recurrence, and Follow-up of Systemic Therapies
Lymph Nodes
Interreader agreement for lymph nodes was in general good to very good for both 18F-DCFPyL and 18F-PSMA-1007. However, a consistently lower agreement, for both weighted κ and percentage of agreement, was found for detection of suspected pelvic lymph nodes and lymph nodes at any localization with 18F-PSMA-1007. No statistically significant differences were found between 18F-DCFPyL and 18F-PSMA-1007 for detection of suspected lymph nodes (Table 2). However, with 18F-PSMA-1007 more lymph nodes showed low-level uptake comparable to blood-pool activity, matching score 1b or 2 (benign or likely benign) (Supplemental Fig. 2). For most anatomic regions, significantly more lymph nodes with low uptake were found with 18F-PSMA-1007 than with 18F-DCFPyL (P < 0.0005–0.037).
Bone Lesions
Regarding suspected bone lesions, almost perfect interreader agreement was found at all localizations for 18F-DCFPyL. For 18F-PSMA-1007, however, consistently lower agreement (both weighted κ and percentage of agreement) was found for lesions in the thoracic region and whole skeleton and to a lesser extent for suspected bone lesions in the pelvis (Fig. 2). Both readers scored a significantly greater number of equivocal bone lesions in the thoracic region with 18F-PSMA-1007 (14%–33% and 3%–4% for 18F-PSMA-1007 and 18F-DCFPyL, respectively) at the expense of the number of scans without bone lesions (44%–62% for 18F-PSMA-1007 and 76%–78% for 18F-DCFPyL). The same was found for bone lesions at any localization (equivocal: 14%–33% for 18F-PSMA-1007 and 3%–4% for 18F-DCFPyL; no bone lesions: 30%–44% for PSMA 1007 and 64%–66% for 18F-DCFPyL) (Table 2; Fig. 3). Reader 2 also scored a significantly greater number of equivocal pelvic lesions (P = 0.027), whereas for reader 1 no statistically significant difference was found (P = 0.053). Categorization according to disease stage shows that a significantly greater number of equivocal lesions were found with 18F-PSMA-1007 at primary staging and biochemical recurrence (P = 0.001 and 0.003, respectively), also at the expense of the number of patients without bone lesions (Table 3).
Visceral Lesions
Visceral lesions were found in 3% of patients by both readers for both 18F-PSMA-1007 and 18F-DCFPyL, whereas equivocal lesions were found in 5%–6% of patients with 18F-DCFPyL and 8% of patients with 18F-PSMA-1007 (Table 2). No statistical differences were found between 18F-PSMA-1007 and 18F-DCFPyL for detection of other lesions. All patients who showed suspected lesions at other locations had extensive metastasized disease in lymph nodes and skeleton.
Equivocal Bone Lesions
Three patients scanned with 18F-DCFPyL (1 primary staging, 1 biochemical recurrence, and 1 therapy follow-up) and 26 scanned with 18F-PSMA-1007 (18 primary staging, 6 biochemical recurrence, and 2 therapy follow-up) had equivocal bone lesions as the only indication of possible metastasized disease. Clinical follow-up of these patients showed definite proof of nonmetastasized disease in 0 and 16 (62%) patients scanned with 18F-DCFPyL and 18F-PSMA-1007, respectively, whereas 1 (33%) and 2 (8%) showed proof of metastasized disease, and no conclusive follow-up was present in 2 (67%) and 8 (31%). Three patients had histopathologic verification of equivocal bone lesions on 18F-PSMA-1007; none of these lesions were proven to be malignant (Fig. 4). Histopathology showed benign etiologies: aspecific necrosis, an aspecific lytic bone lesion with reactive changes, and normal bone tissue.
Equivocal bone lesions in right clavicula (A) and ribs (B and C) of clinical relevance for primary staging on 18F-PSMA-1007 PET/CT. Tumor characteristics were cT3a, Gleason score of 7, and initial PSA of 15.2 ng/mL in A; cT2, Gleason score of 8, and initial PSA of 8.0 ng/mL in B; and cT3a, Gleason score of 6, and initial PSA of 3.7 ng/mL in C. Additional MRI could not exclude bone metastasis in A; no further imaging was performed in B and C. Histopathologic biopsy revealed no malignancy in any of these 3 patients: aspecific necrosis was found in A, aspecific lytic bone lesion with reactive changes in B, and normal bone tissue in C. Ultimately, all 3 patients were staged N0M0.
DISCUSSION
Although great equality was found between 18F-DCFPyL and 18F-PSMA-1007 for interreader agreement and for detection of suspected prostate cancer lesions, there were 2 differences that may be of clinical relevance.
The first was in the prostatic region; identification of lesions in this region is of particular clinical importance for patients with biochemical recurrence. The subanalysis according to clinical stage showed that, especially in this category of patients, 18F-PSMA-1007 detected significantly more suspected lesions than 18F-DCFPyL, although the number of patients in this category was limited (n = 21). That finding could be the result of the lower urinary secretion of this radiopharmaceutical, allowing for better characterization of intraprostatic lesions or lesions in the prostatic fossa after prostatectomy.
Second, equivocal bone lesions were found more frequently with 18F-PSMA-1007, resulting in lower interreader agreement. The greatest difference between readers was in the classification of rib uptake. Both readers acknowledged high uptake in the ribs in many patients scanned with 18F-PSMA-1007. Reader 1 interpreted this uptake as probably benign (PSMA RADS 2) in a larger proportion of patients than did reader 2, who scored a larger proportion as equivocal (PSMA RADS 3a–3d). Reader 1 argued that the absence of lesions at other sites in the skeleton made bone metastases in the ribs unlikely; however, reader 2 scored these rib lesions as equivocal based purely on the increased activity in the ribs. Reader 2 argued that solitary bone metastases in the ribs are encountered in prostate cancer patients, although (as far as is known today) normally not as frequently as is observed with 18F-PSMA-1007. The PSMA RADS reading criteria allow for interpretation differences, since no hard cutoffs to classify tracer uptake are given. Although allowing for differences in interpretation is scientifically less desirable, such differences continuously exist in clinical practice, and these data therefore reflect the present discussion around this topic in daily practice. Findings of equivocal PSMA-avid bone lesions may be clinically relevant and account for diagnostic dilemmas, such as when there are no other metastatic lesions or when the equivocal findings distinguish between oligo- and polymetastatic disease. In these cases, the true nature of these equivocal findings may alter patients’ management and indicate the need for further diagnostic procedures.
Given that the study was retrospective, it had the drawback that histopathologic confirmation was lacking for almost all lesions. However, clinical follow-up of patients with equivocal bone lesions as the only indication of possible metastasized disease showed that in most patients (at least 16/24) with these abnormalities on 18F-PSMA-1007, metastasized disease could be excluded. However, metastasized disease was proven in only 2 of 26 patients. These results indicate that equivocal bone findings with 18F-PSMA-1007 PET/CT are of little clinical value and should not cause withholding of treatment options with curative intent.
As another drawback of the study, because 18F-DCFPyL and 18F-PSMA-1007 were compared between patients, some findings may be attributable to differences between cohorts rather than to differences between radiopharmaceuticals. However, to diminish these influences, we used 2 large cohorts comprising matched-pair patients. Unfortunately, the databases were not large enough to enable pairing by all potentially relevant clinical characteristics that may interfere with the pretest likelihood of detecting suspected lesions. Matching of PSA was done instead of matching of Gleason score or T stage, since there are indications that 18F-PSMA-PET/CT positivity for metastases is best predicted by the serum PSA value (19).
The findings in this study are in line with findings by Dietlein et al. in an intrapatient comparison of 18F-PSMA-1007 with 18F-DCFPyL, 68Ga-PSMA-11, or 18F-JK-PSMA-7 (20). In a cohort of 27 patients, an improved characterization of prostate lesions with 18F-PSMA-1007 was shown (P = 0.024) at the expense of the interpretability of skeletal lesions. 18F-PSMA-1007 showed significantly (P = 0.0006) more aspecific medullary focal uptake. However, in another study, which included 12 patients scanned with both 18F-DCFPyL and 18F-PSMA-1007, both radiopharmaceuticals identified the same lesions; the appearance of equivocal skeletal uptake was not mentioned (15).
Recently, Rauscher et al. performed a matched-pair analysis of 68Ga-PSMA-11 and 18F-PSMA-1007 and found an almost 5 times higher prevalence of PSMA-ligand–positive findings attributed to a benign origin, including among other bone lesions and lymph nodes (21). In this study, as in our present study, the lack of histopathologic confirmation represents a major limitation. To our knowledge, ours was the first study comprising a large cohort of patients assessing the differences between 2 widely used 18F-PSMA radiopharmaceuticals.
Nonspecific skeletal 18F-PSMA-1007 uptake has an unknown mechanism and shows great heterogeneity between patients (Fig. 1). It might be hypothesized that radiolysis results in free 18F-fluorine; however, quality control reports from our cyclotron facility mention concentrations of free 18F-fluorine below 2% at 8 h after synthesis of 18F-PSMA-1007. Furthermore, the aspecific skeletal uptake was not seen in patients whose prostate cancer was scanned with 18F-sodium fluoride (22). Therefore, it is unlikely that the uptake is explained by the presence of free 18F-fluorine due to radiolysis. The literature comparing PSMA tracers with 68Ga and 18F has suggested that the higher number of equivocal lesions found with 18F-PSMA-1007 might be explained by the lower positron energy (which improves spatial resolution), longer half-life, and generally higher injected activities of 18F than of 68Ga (21). However, since this study compared 2 different 18F-labeled PSMA tracers, it can be concluded that the differences in physical properties of 68Ga and 18F do not offer a good explanation for the number of equivocal lesions found with 18F-PSMA-1007. It is known that PSMA uptake may appear in several benign (bone) lesions (23); therefore, one hypothesis is that higher affinity of 18F-PSMA-1007 for the PSMA receptor, as shown in preclinical studies, may result in a higher signal from these benign lesions (24). Another possibility is that 18F-PSMA-1007 is metabolized (e.g., in the liver before excretion in the bile), resulting in radiolabeled substances that accumulate at specific sites in the skeleton, although there is no scientific underpinning for this hypothesis.
Given the findings in the present study and in the available literature, it can be concluded that there are differences between 18F-DCFPyL and 18F-PSMA-1007 that may have clinical consequences. Although there are clues that 18F-DCFPyL may be more appropriate when information about uptake in the prostate is of no clinical value (e.g., in primary staging of prostate cancer that is already histopathologically proven), and although 18F-PSMA-1007 may be more appropriate when detection of abnormalities in the prostate or prostatic fossa are most clinically relevant, there is too little evidence to support recommendations on which tracer should be used. Further studies are needed to verify the clues found in this study and in the available literature, especially studies to analyze the true nature of the equivocal bone lesions frequently found with 18F-PSMA-1007 and the apparent better ability of 18F-PSMA-1007 to detect lesions in the prostate and prostatic region.
CONCLUSION
Great equality was found between 18F-DCFPyL and 18F-PSMA-1007; however, some differences were observed that may be of clinical relevance. 18F-PSMA-1007 detected prostatic lesions and prostatic fossa lesions in a higher proportion of patients, whereas 18F-DCFPyL showed fewer equivocal skeletal lesions and higher interreader agreement for skeletal lesions. These differences encourage further studies to evaluate their true clinical impact, as they may have consequences for selection of the proper PSMA-targeting radiopharmaceutical.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Are differences in the biologic behavior of different PSMA tracers clinically relevant to interreader agreement and to the detection rate for suspected malignant lesions?
PERTINENT FINDINGS: Interreader agreement was generally better for 18F-DCFPyL than for 18F-PSMA-1007, particularly regarding bone lesions. A statistically greater number of suspected lesions was found in the prostate or prostatic fossa with 18F-PSMA-1007, especially in the biochemical-recurrence subcohort (P = 0.007–0.029), whereas 18F-DCFPyL found a statistically lower number of equivocal bone lesions, particularly in the biochemical-recurrence (P = 0.003–0.013) and primary-staging (P = 0.001–0.065) cohorts
IMPLICATIONS FOR PATIENT CARE: Although more studies are needed to further explore the exact clinical implications of the present findings, these findings may allow for better selection of PSMA-targeted radiopharmaceuticals and therefore increase the diagnostic power of PSMA PET/CT.
Footnotes
Published online February 5, 2021.
- © 2021 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication October 20, 2020.
- Accepted for publication January 13, 2021.