Abstract
Neuroendocrinelike transdifferentiation of prostate cancer adenocarcinomas correlates with serum levels of chromogranin A (CgA) and drives treatment resistance. The aim of this work was to evaluate whether CgA can serve as a response predictor for 177Lu-prostate-specific membrane antigen 617 (PSMA) radioligand therapy (RLT) in comparison with the established tumor markers. Methods: One hundred consecutive patients with metastasized castration-resistant prostate cancer scheduled for PSMA RLT were evaluated for prostate-specific antigen (PSA), lactate dehydrogenase (LDH), and CgA at baseline and in follow-up of PSMA RLT. Tumor uptake of PSMA ligand, a known predictive marker for response, was assessed as a control variable. Results: From the 100 evaluated patients, 35 had partial remission, 16 stable disease, 15 mixed response, and 36 progression of disease. Tumor uptake above salivary gland uptake translated into partial remission, with an odds ratio (OR) of 60.265 (95% confidence interval [CI], 5.038–720.922). Elevated LDH implied a reduced chance for partial remission, with an OR of 0.094 (95% CI, 0.017–0.518), but increased the frequency of progressive disease (OR, 2.717; 95% CI, 1.391–5.304). All patients who achieved partial remission had a normal baseline LDH. Factor-2 elevation of CgA increased the risk for progression, with an OR of 3.089 (95% CI, 1.302–7.332). Baseline PSA had no prognostic value for response prediction. Conclusion: In our cohort, baseline PSA had no prognostic value for response prediction. LDH was the marker with the strongest prognostic value, and elevated LDH increased the risk for progression of disease under PSMA RLT. Elevated CgA demonstrated a moderate impact as a negative prognostic marker in general but was explicitly related to the presence of liver metastases. Well in line with the literature, sufficient tumor uptake is a prerequisite to achieve tumor response.
Androgen deprivation therapy followed by novel androgen-axis drugs such as enzalutamide and abiraterone have been shown to improve overall survival in patients with prostate cancer (1,2). For patients with metastasized castration-resistant prostate cancer who have exhausted standard therapies, prostate-specific membrane antigen therapy became a possible last-line therapy (3).
De novo neuroendocrine prostate cancer is uncommon (∼1%); however, after multiple treatment lines, up to 40% of patients initially diagnosed with adenocarcinoma present a conversion or transdifferentiation of prostate adenocarcinoma into neuroendocrinelike cells, also named treatment-related neuroendocrine prostate cancer (4,5). During this tumor evolution to high-grade prostate cancer, pluripotent tumor stem cells undergo epithelial–mesenchymal transition, and the increasing number of neuroendocrine cells, which are not regulated by androgens, contributes to acquired resistance against antihormonal therapies (6–8). The increase of neuroendocrine cells between early- and advanced-stage prostate cancer may result in elevated serum chromogranin A (CgA) levels.
CgA is an acidic glycoprotein, commonly overexpressed by neuroendocrine cells and released to the blood by secretory granules (6). In patients with prostate cancer, CgA is released by primary tumors and also metastases and is considered a possible surrogate reflecting the increase in neuroendocrinelike cells in advanced-stage metastasized castration-resistant prostate cancer (9). Several studies described high CgA levels to be related to high-grade prostate cancer (10), advanced-stage disease with a poor prognosis, resistance against enzalutamide and abiraterone (6,7), and reduced overall survival (8,11). Neuron-specific enolase, also suggested as a potential neuroendocrine biomarker, was recently reported to have lower sensitivity and specificity than CgA (8,12).
Similarly to neuroendocrine transdifferentiation, prostate-specific membrane antigen overexpression was also reported to increase in advanced-stage, hormone-resistant tumors (13). Until now, no data about prostate-specific membrane antigen expression in treatment-related neuroendocrine prostate cancer have been available. However, for treatment-related neuroendocrine prostate cancer, resistance mechanisms to subsequent treatments (radiation therapy, androgen deprivation therapy, second-generation antihormonal therapy, and chemotherapy) are known (5,7). This knowledge leads to the question of whether partial neuroendocrine transdifferentiation might also increase resistance against 177Lu-prostate-specific membrane antigen 617 (PSMA) radioligand therapy (RLT).
The aim of this study was to evaluate whether CgA is a potential prognostic marker in metastasized castration-resistant prostate cancer patients treated with PSMA RLT and how this biomarker may perform in comparison to other established tumor markers such as prostate-specific antigen (PSA) and lactate dehydrogenase (LDH).
MATERIALS AND METHODS
Patients
Patients with metastasized castration-resistant prostate cancer after exhausting (or being considered ineligible for) approved treatment options were treated with PSMA RLT under the conditions of the updated Declaration of Helsinki, article 37, “Unproven Interventions in Clinical Practice.” One hundred consecutive patients who had received PSMA were selected for this study. With awareness of receiving an experimental therapy, all patients gave written informed consent. The retrospective data evaluation was approved by our institutional review board.
Patient history and current drug medication plan were explored for known confounders of CgA serum levels; that is, patients with chronic atrophic gastritis (14), long-term use of proton-pump inhibitors (15), reduced renal function (16), and chronic heart failure (17). All 100 patients and a subgroup of 65 patients without CgA confounders were evaluated separately. The characteristics of both groups are presented in Table 1.
Patient Characteristics
The treatment concept followed the national consensus recommendation for the use of PSMA RLT (18), with treatment cycles conducted every 8 wk. According to the consensus guidelines (18), PSMA treatment was reevaluated after 2 cycles. If patients were referred to our department and the uptake in the main tumor sites was too weak (uptake in most metastases at or below liver level), treatment was discontinued even after 1 cycle. In cases of an insufficient tumor response or a complete tumor response, other treatment concepts using different nuclides were initiated or therapy was stopped, respectively. Baseline blood work was checked before each cycle of PSMA RLT, and observational laboratory reports and results in between the treatment cycles were used for response assessment. As a clinical standard, LDH, PSA, and CgA were determined.
Lab Tests
Lab analysis for LDH was performed in an ADVIA Chemistry XPT (Siemens) using an enzyme-based method by catalysis of lactate to pyruvate. Measurement level was based on changes in wavelength at 340 nm. The reference blood level was less than 342 U/L. PSA was measured using a Zentaur XPT (Siemens) system with a chemiluminescent immunoassay (sandwich immunoassay). The reference blood level was less than 4 μg/L. Serum CgA was measured in a Kryptor Compact Plus (Thermo Fisher Scientific) using an immunofluorescence assay by means of trace technology for a cryptate-antibody-donator complex for immunocomplex measurement. The reference level was less than 84.7 ng/mL.
Biochemical response was defined as a PSA drop of more than 50%, biochemically stable disease was defined as a PSA of between −50% and +30%, and progression of disease was defined as a PSA increase of more than 30% in comparison to baseline.
PSMA Imaging
Posttherapeutic scintigraphy 20–24 h after injection (planar anterior and posterior whole-body scans in a dual-head γ-camera [Hawkeye Millennium; GE Healthcare] with medium-energy parallel-hole collimator, a scan speed of 15 cm/min, and the 208 keV ± 10% [187–228 keV] photo peak window) was evaluated at the first cycle. Two visually evaluable reference levels in grayscale were set (1: background uptake of the liver; 2: uptake of the salivary glands). If patients had a mainly tumor-related PSMA-617 uptake at or below liver level in the predominant tumor lesions, no further PSMA treatments were provided. At every cycle we performed a clinical examination, lab tests, and the posttherapeutic scintigraphy (20–24 h after injection). PSMA imaging was 99mTc-PSMA SPECT/CT in 15 patients, 68Ga-PSMA11 PET/CT in 75, 18F-PSMA1007 PET/CT in 8, and 18F-DCFPYL in 2; restaging was performed with the modality already available at baseline.
Image Interpretation
On the basis of visual interpretation of baseline imaging, tracer uptake was categorized as intense, heterogeneous, or faint. Semiquantitative tailoring into the 3 groups was performed by comparison of the average tumor uptake on baseline (at the first cycle) posttherapeutic scintigraphy to liver background and salivary gland background. Intense uptake was defined as uptake semiquantitatively above the salivary gland level on posttherapeutic scintigraphy. Heterogeneous uptake was defined as uptake above the liver grayscale level but below the salivary gland grayscale level. Faint uptake was defined as uptake at or below the liver grayscale level.
For statistical testing, only the categories partial remission and progression of disease were used. Partial remission was defined by a reduction of previously known PSMA-positive lesions and no new lesions. Progression was defined as the occurrence of new lesions or an increase in all known lesions.
For visual interpretation, we additionally introduced an arbitrary category named mixed response that was used for patients without new lesions, PSA of between −50% and +30%, but simultaneously presenting with increasing and decreasing old lesions.
Statistical Analysis
The results were initially assessed using Excel (version 2007; Microsoft Corp.). The main statistical analysis was then performed using function “glm” in R (version 3.4.0; R Foundation for Statistical Computing). The statistical significance level was set at a P value of less than 0.05.
Both a univariable and a multivariable logistic regression analysis were once performed on the dataset of 100 patients (with a total of 211 treatment cycles) and once on the subgroup of 65 patients without CgA confounders, investigating the effect of 3 independent variables (PSA before PSMA RLT, CgA before PSMA RLT, and LDH before PSMA RLT) as well as tracer uptake on the dependent variables partial remission and progression of disease. Tracer uptake was included in the form of 2 dummy variables, defined as heterogeneous uptake (yes vs. no) and intense versus faint uptake as the standard. To correct for skewed distributions, the quantitative variables were evaluated on a logarithmic scale (log 10 for PSA and log 2 for LDH and CgA). Different log transformations were used because of the different reference levels of the evaluated lab parameters and the probability of whether a 2-fold or a 10-fold increase represents a clinically meaningful difference. Results were expressed as odds ratios (ORs), with corresponding 95% confidence intervals (CIs) and P values. Additional univariable logistic regression analyses were performed in the same way to predict the presence of metastases at different locations depending on level of CgA only.
RESULTS
One hundred patients and a subgroup of 65 patients with, in total, 211 cycles of PSMA RLT were evaluated in a multivariable analysis (Table 1). Initial LDH, CgA, and PSA before RLT were selected for lab evaluation regarding the clinical outcome (either partial remission [yes vs. no] or progressive disease [yes vs. no]). Overall outcome data are presented in Table 2. Lab evaluation for univariable and multivariable analyses is presented in Tables 3–5.
Numbers of Patients with Respective Characteristics
Lab Evaluation Regarding the Clinical Outcome “Partial Remission” (n = 100), Evaluated by Univariable and Multivariable Logistic Regression Analysis
Lab Evaluation Regarding the Clinical Outcome “Progression of Disease” (n = 100)
Lab Evaluation Regarding the Clinical Outcome “Progression of Disease” Without Confounders for CgA Measurement (Patients Without Evaluable Confounders As Pantoprazole or Gastritis or Renal Failure) (n = 65)
Of the 100 evaluated patients, 35 had partial remission, 16 stable disease, 15 mixed response, and 36 progression of disease (Table 2).
LDH values before PSMA RLT were significant for outcome prediction. Using the univariable model, LDH before PSMA RLT showed an OR of 0.117 (95% CI, 0.031–0.441; P = 0.0015) regarding the clinical outcome. Using the multivariable model, LDH also indicated a reduced risk, with an OR of 0.094 (95% CI, 0.017–0.518; P = 0.006) for the complete group (n = 100). Elevated LDH had an increased risk for progression in the univariable model, with an OR of 3.239 (95% CI, 1.755–5.979; P = 0.0002), and in the multivariable model, with an OR of 2.717 (95% CI, 1.391–5.304; P = 0.003). Baseline LDH values in comparison to outcome (PSA at restaging) are shown in Figure 1.
LDH at first cycle of PSMA RLT in comparison to percentage change in PSA at restaging. Dashed line depicts upper limit of LDH (342 U/L).
About the prognosis for partial remission, CgA showed no significance for outcome prediction (P = 0.1657 for the univariable model and P = 0.8501 for the multivariable model). Regarding progressive disease, CgA showed elevated risk in the univariable model, with an OR of 3.385 (95% CI, 1.597–7.175; P = 0.0015), and in the multivariable model, with an OR of 3.089 (95% CI, 1.302–7.332; P = 0.011), in the subgroup without confounders (n = 65). For the group of all patients, the effect was weaker and barely not significant using the multivariable analysis (P = 0.08) but remained significant for the univariable model, with an OR of 1.597 (95% CI, 1.144–2.229; P = 0.006).
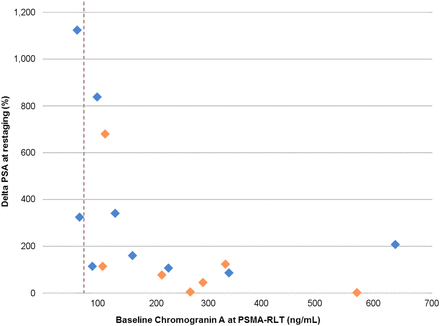
Elevated CgA before RLT had an increased risk for organ metastases in the liver, with an OR of 1.861 (95% CI, 1.048–3.305; P = 0.0341), for all patients. In the subgroup, results stayed robust for organ metastases in the liver, with an OR of 1.527 (95% CI, 1.034–2.255; P = 0.0333). For all other organ or soft-tissue metastases, there was no significantly elevated risk associated with CgA (Tables 6 and 7). Baseline CgA values in comparison to outcome (PSA at restaging) for the group with progression are shown in Figure 2.
OR and 95% CI for Presence of Metastases Depending on Level of CgA: Subgroup of Patients Without Evaluable Confounders Such as Pantoprazole or Gastritis or Renal Failure (n = 65)
OR and 95% CI for Presence of Metastases Depending on Level of CgA: All Patients (n = 100)
CgA at first cycle of PSMA RLT in comparison to percentage change in PSA at restaging for group with progression of disease. Dashed line depicts upper limit of CgA (84.7 ng/mL). Blue diamonds are patients without potential confounders. Orange diamonds are patients with potential confounders.
PSA level before RLT was not significant using the multivariable analysis for prediction of either partial remission (P = 0.0938) or progression of disease (P = 0.436). No significant odds can be provided, therefore (Tables 3–5). For the univariable model, significant odds can be provided for partial remission, with an OR of 0.461 (95% CI, 0.265–0.802; P = 0.0061), and for progression of disease, with an OR of 1.765 (95% CI, 1.043–2.985; P = 0.0342). For imaging evaluation, an intense tumor uptake (above salivary gland level) was significant for prediction of partial remission. The ORs for intense uptake and partial remission were 18.0 (95% CI, 2.230–145.3119; P = 0.0067) for the univariable model and 60.265 (95% CI, 5.038–720.922; P = 0.001) for the multivariable model. For progression of disease, intense tumor uptake had a protective OR of 0.230 (95% CI, 0.075–0.703; P = 0.0099) for the univariable model and 0.165 (95% CI, 0.044–0.624; P = 0.0079) for the multivariable model (Tables 3–5). However, not all patients with intense tumor uptake (n = 50) had a partial remission (n = 30) (Table 2). A waterfall graph of all 100 patients (Fig. 3) compares the PSA response to baseline and to the results of posttherapeutic scintigraphy. Thirty-one patients had a PSA drop of 50% or more in comparison to baseline. Fifty-one patients had any PSA response. Two patients had no PSA response and remained at a stable PSA level although imaging showed a decreased tracer uptake. Thirty-one patients had PSA progression, 26 of whom had PSA progression of more than 30%. Twenty-six of 51 patients had a PSA response and a response on posttherapeutic scintigraphy. Twenty-one of 51 patients had a PSA response and stable disease or a mixed response on imaging. Four of 51 patients had progression on imaging although a PSA response was detectable. Thirteen of 31 patients had stable disease or a mixed response with a PSA rise at restaging. Seventeen of 31 patients had visual and biochemical progression. One of 31 patients had a visual response on posttherapeutic scintigraphy although he had a PSA rise of more than 100%; in this case, we assume a PSMA-negative tumor phenotype.
Waterfall graph of PSA response compared with color-coded response in imaging.
DISCUSSION
PSMA RLT is an emerging approach to treat advanced-stage prostate cancer patients. The aim of this study was to evaluate whether CgA has a prognostic value for response prediction and how this biomarker compares with the established tumor markers PSA and LDH.
PSMA PET or SPECT is routinely performed in advance of PSMA RLT to select only patients positive for the target receptor. However, only a moderate correlation (r = 0.61) between PSMA PET and tumor uptake during therapy was found (19). This result might be explained by the different structures of the imaging tracers that have been optimized for rapid tumor targeting but are only surrogates for PSMA-617, which has been optimized for therapy. In addition, no PSMA imaging agent has yet been approved, but several compounds are under investigation. To relay on a common standard, we semiquantitatively assessed (tumor-to-salivary gland, tumor-to-liver) PSMA uptake with the emission scan of the first treatment cycle. Homogeneously high uptake was a prerequisite to achieve partial remission, and the high OR observed demonstrates the appropriateness of this approach. These findings are well in line with expectations and previously published studies (19,20).
We observed that baseline PSA, which is considered to reflect baseline tumor volume, had no significance for response prediction. This result is in line with previously published data, as not the baseline PSA but the PSA response seems to be more meaningful (21).
The strongest value for response prediction was baseline LDH, a cytosolic enzyme that is released to serum in correlation with cell turnover and thus reflects an unspecific surrogate for the aggressiveness of tumor growth. This result is well in line with former studies, as LDH was previously described to be a relevant marker for response prediction (21,22) and associated with progression-free survival (21).
For the patients without CgA confounders, an elevated baseline CgA was related to an increased risk for progression (OR, 3.09; P < 0.05). However, it was no more significant (P = 0.08) if the patients with confounders were also included; this inclusion rendered 35 of 100 patients unevaluable. Nevertheless, the OR reflects only an average risk; individual patients with elevated baseline CgA could also achieve partial remission.
In both evaluated cohorts (with and without CgA confounders), a statistically significant association between CgA and the presence of liver metastases was found. There were no relationships between CgA and metastases in lung, brain, or other organs.
Although the role of neuroendocrine cells in prostate cancer is not completely understood, paracrine effects and stimuli on growth regulation are well known (23), and overexpressing the antiapoptotic protein survivin leads to resistance to programmed cell death (5,24). With an increase of neuroendocrine cells in late-stage metastasized castration-resistant prostate cancer (initially more scattered neuroendocrine cells or small nests among the predominant epithelial cells in early stages) (25–27), this pattern might become more relevant in liver metastases, as poor differentiation and neuroendocrine differentiation are more present (28,29). Therefore, we assume a higher incidence of PSMA-negative tumor phenotype at these hepatic tumor sites. For clinical practice, an elevated CgA and nonresponse to PSMA RLT might indicate a PSMA-negative tumor, especially in the liver. However, other variables also need to be taken in account, such as mutations in tumor suppression genes, because patients without liver metastasis and with normal CgA also had a nonresponse to PSMA treatment (30). This finding implies that in patients with a high baseline CgA, special focus should be given to liver imaging.
The main limitation of the present evaluation is its single-center, retrospective design. Furthermore, CgA elevation is described not only for neuroendocrine tumors but also for chronic atrophic gastritis, use of proton pump inhibitors, renal failure, and chronic heart failure (14–17) and might be present without pathologic cause. Especially, proton pump inhibitors are widely used in patients after treatment with second-generation antihormonal therapies (abiraterone acetate and enzalutamide), and concomitant treatment with steroids (prednisone) might cause a CgA elevation without any existing pathology. Also, it should be noted that for the absolute values of tumor markers, the reference range is defined as the mean ± 2 SDs, and 2.5% of the healthy population will physiologically have values above the upper limit (31). Because PSMA RLT is a last-line therapy and patients usually received second-generation antihormonal treatments with concomitant antacid, our results for CgA should be cautiously transferred into clinical practice and a validation of our results in a prospective design should be performed.
CONCLUSION
Although CgA demonstrated some potential as a negative prognostic biomarker, it was found to be inferior to LDH, and its clinical value is remarkably hampered by numerous confounders that can nonspecifically increase its serum level in more than one third of patients. In contrast to other markers, elevated CgA is associated with an increased risk for liver metastasis and might be used to guide liver-specific imaging.
DISCLOSURE
Uwe Haberkorn, Clemens Kratochwil, Walter Mier, and Klaus Kopka hold a patent for PSMA-617. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is response in prostate cancer to PSMA RLT predictable by lab measurement before the first cycle?
PERTINENT FINDINGS: In this retrospective single-center evaluation, 100 patients under PSMA RLT were evaluated by comparing their baseline lab values before RLT with their clinical outcome. The most beneficial constellation of lab parameters was a high tumor uptake of tracer-associated activity in combination with a low LDH and a low CgA before PSMA RLT.
IMPLICATIONS FOR PATIENT CARE: If LDH or CgA was elevated before PSMA RLT, patients had an elevated risk for nonresponse and progression of disease under therapy and an elevated risk for liver metastases.
Footnotes
Published online Oct. 25, 2019.
- © 2020 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication May 22, 2019.
- Accepted for publication September 27, 2019.