Abstract
Our objective was to assess the feasibility and accuracy of Cerenkov luminescence imaging (CLI) for assessment of surgical margins intraoperatively during radical prostatectomy. Methods: A single-center feasibility study included 10 patients with high-risk primary prostate cancer (PC). 68Ga-prostate-specific membrane antigen (PSMA) PET/CT scans were performed followed by radical prostatectomy and intraoperative CLI of the excised prostate. In addition to imaging the intact prostate, in the first 2 patients the prostate gland was incised and imaged with CLI to visualize the primary tumor. We compared the tumor margin status on CLI to postoperative histopathology. Measured CLI intensities were determined as tumor-to-background ratio. Results: Tumor cells were successfully detected on the incised prostate CLI images as confirmed by histopathology. Three of 10 men had histopathologically positive surgical margins (PSMs), and 2 of 3 PSMs were accurately detected on CLI. Overall, 25 (72%) of 35 regions of interest proved to visualize a tumor signal according to standard histopathology. The median tumor radiance in these areas was 11,301 photons/s/cm2/sr (range, 3,328–25,428 photons/s/cm2/sr), and median tumor-to-background ratio was 4.2 (range, 2.1–11.6). False-positive signals were seen mainly at the prostate base, with PC cells overlaid by benign tissue. PSMA immunohistochemistry revealed strong PSMA staining of benign gland tissue, which impacts measured activities. Conclusion: This feasibility showed that 68Ga-PSMA CLI is a new intraoperative imaging technique capable of imaging the entire specimen’s surface to detect PC tissue at the resection margin. Further optimization of the CLI protocol, or the use of lower-energy imaging tracers such as 18F-PSMA, is required to reduce false-positives. A larger study will be performed to assess diagnostic performance.
- Cerenkov luminescence imaging
- radioguided surgery
- prostate cancer
- margin assessment
- radical prostatectomy
See an invited perspective on this article on page 1498.
Radical prostatectomy (RPE) is one of the primary treatment options for men with localized prostate cancer (PC). The aim of the procedure is complete resection of the prostate without positive surgical margins (PSMs) (1). The pentafecta outcome criteria for RPE include continence, potency, lack of early complications, lack of biochemical recurrence, and lack of PSMs and should be fulfilled whenever oncologically possible (2).
Incomplete removal of the cancer tissue during RPE may be associated with poorer patient outcomes (3). A PSM increases the need for adjuvant radiotherapy, the likelihood of recurrence, and the chances of PC-related mortality by a factor of 3 (3). PSMs of more than 3 mm are known to increase the risk of biochemical recurrence (4). Similarly, men with persistently elevated PSA levels of at least 0.1 ng/mL more than 6 wk after RPE show less favorable cancer control and survival rates over time (5–7).
Next to preoperative MRI for local staging of disease, and nomograms for the prediction of extracapsular extension, the use of intraoperative frozen-section analysis can facilitate the surgeon’s decision concerning preservation of functional structures such as the neurovascular bundles (1,8–10).
Schlomm et al. described significantly less frequent PSMs of 15% versus 22% for all stages and 21% versus 32% for extraprostatic disease, suggesting that systematic application of intraoperative frozen-section analysis has the potential to significantly increase nerve sparing and to reduce PSMs in RPE (8). Other studies, however, demonstrated a high false-negative rate of intraoperative frozen-section analysis, potentially resulting in unjustified nerve-sparing surgery, and eminent time consumption (11). Consequently, there is a wish to be able to accurately detect malignant areas in real time during RPE to ensure that the PC is completely removed. Molecular imaging with modern radiopharmaceuticals that target the prostate-specific membrane antigen (PSMA) can be used for highly specific and sensitive oncologic diagnostic imaging, including initial staging of PC (12–15). PSMA PET is an imaging technique that targets PSMA on PC cells, mostly with 68Ga-labeled and 18F-labeled PET agents (16).
In recent trials, PSMA PET demonstrated high detection rates for lymph nodes metastases and a positive predictive value for initial staging of PC (17–20). Maurer et al. described the advantage of PSMA-radioguided surgery for pelvic lymph node dissection, visualizing even small lymph node metastasis (21).
PET imaging agents also emit optical photons via a phenomenon called Cerenkov luminescence, enabling optical molecular imaging in the form of Cerenkov luminescence imaging (CLI) (22). CLI and PET are directly correlated, as both techniques measure photons: PET measures the γ-annihilation photons, and CLI measures the Cerenkov photons (23). Cerenkov photons are emitted by a charged particle (positron or electron) when traveling through a dielectric medium at a faster speed than the velocity of light in that medium. Although Cerenkov luminescence has a broadband wavelength spectrum, it comprises predominantly ultraviolet and blue light, and as these short wavelengths are highly attenuated in biologic tissue, CLI is limited to detection of signals emitted in superficial tissue layers (∼3 mm). In contrast to PET, CLI is unable to detect photons emitted by more deeply located tissues or tumors (24,25).
The clinical feasibility and safety of CLI were demonstrated in a small number of breast cancer patients using 18F-FDG (24). In that study, good agreement between CLI and histopathology was found for margin distance and invasive cancer tumor size.
To our knowledge, there are no published data available, to date, regarding CLI in PC using a PSMA-labeled tracer. Therefore, the aim of our prospective study was to analyze the feasibility and diagnostic accuracy of intraoperative CLI to detect PSMs in a cohort of 10 men undergoing RPE.
MATERIALS AND METHODS
Patient Recruitment and Patient Preparation on Day of Surgery
Between February 2018 and June 2019, patients with histologically confirmed PC on systematic or MRI-targeted prostate biopsy were recruited at University Hospital Essen after discussion by the local multidisciplinary tumor board and procurement of written informed consent. This study received formal Ethical Committee approval (19-8749-BO). Exclusion criteria were previous prostate surgery or radiotherapy, known distant metastases, and contraindications to surgery.
On the day of surgery, 68Ga-PSMA-11 (1.8–2.2 MBq/kg of body weight) was injected intravenously in patients scheduled to undergo RPE (26). At 45–60 min after injection, the PET/CT scan was performed and assessed by experienced nuclear medicine physicians. If any cases of high-volume metastatic disease had been discovered on PET/CT, same-day surgery would have been cancelled.
RPE and Intraoperative CLI
After PET/CT, RPE of these patients was performed by 2 surgeons ahead of extended pelvic lymph node dissection to minimize any reduction in signal intensity from radiotracer decay during 68Ga-PSMA injection and CLI. An indwelling catheter was immediately inserted in the operating theater before surgery to drain the urine and minimize urinary contamination of the prostate. After excision of the prostate, the specimen was immediately retrieved from the abdomen, wiped to clear blood and fluids, positioned in a specimen tray, and imaged in the LightPath CLI system (Lightpoint Medical Ltd.). The LightPath system captured both a Cerenkov image and a photographic image of the specimen in a lighttight imaging chamber and was positioned in the operating room to enable real-time image analysis by the surgeon. Images were acquired with a total time of 300 s and 8 × 8 pixel binning (27). The prostate gland was imaged in different positions, and a total of 2 or 3 images was necessary to capture all sides of the prostate. After the intact prostate was imaged, the prostate gland was incised in the first 2 patients at the level of the main primary lesion as seen on MRI and was subsequently imaged with CLI to visualize the primary tumor. On CLI completion, the prostatectomy specimen was sent for postoperative histopathologic analysis. Because of the design of this feasibility study, the surgical course remained unaffected by the CLI results, and no further tissue was resected if positive margins were suspected on CLI.
Radiation Safety
After completion of CLI, radiation safety was monitored by accurately quantifying the activity in the excised prostate specimen using a high-purity germanium detector (Canberra). The activity was decay-corrected to the time of excision.
Image Analysis
The Cerenkov luminescence images were analyzed postoperatively in a controlled and standardized analysis environment using PMOD (version 3.204; PMOD Technologies). The mean radiance (photons/s/cm2/sr) was measured by drawing regions of interest (ROIs) on the Cerenkov luminescence images. ROIs were selected in areas showing increased signal intensity (tumor) and no increased signal (tissue background), and tumor-to-background ratios (TBRs) were calculated. Tumor margin assessment on CLI was performed by analyzing elevated signals at the surface of the intact prostate images.
Histopathology and Immunohistochemistry
Histopathologic evaluation was performed adherent to the current German S3-guidelines for PC (28). RPEs were evaluated by expert uropathologists, and typical PC metrics were reported, including Gleason grading in its 2014 revision (29). PSMA immunohistochemistry was performed on an automated Ventana Benchmark Ultra device (Ventana Medical Systems) using PSMA antibody clone 3E6 (Agilent/DAKO) with a 1:50 dilution. Membranous PSMA expression in immunohistochemistry analyses was stratified according to its primary intensity of staining. Moderate and strong intensities were considered positive (30).
RESULTS
In total, 10 patients were included in the presented feasibility study. Their characteristics are displayed in Table 1. Median age at surgery was 72 y, median PSA was 9.04 ng/mL, and median body mass index was 28.6. The median administered 68Ga-PSMA activity was 119 MBq (range, 95–202 MBq). The median time between intravenous injection and the start of surgery was 223 min (range, 153–328 min), and the median time between tracer injection and the beginning of CLI acquisition was 333 min (range, 282–429 min). The median decay-corrected tracer activity at CLI of the prostate was 0.34 kBq/mL (range, 0.13–3.1 kBq/mL; Table 2).
Patient and Oncologic Characteristics
Overview of Injected Activity and Duration Between Injection, Surgery, and CLI per Patient
The incised-prostate images acquired for the first 2 patients showed an elevated signal in the primary lesion as confirmed by histopathology (patient 1, TBR of 2.95; patient 2, TBRs of 4.89 for lesion 1 and 4.26 for lesion 2) (Fig. 1).
Gray-scale photographs overlaid with Cerenkov signals for incised prostate gland specimen of patients 1 (A) and 2 (B). In each image, ROIs are encircled. Incision is marked with dotted line. Light blue ROI (area of empty specimen tray) and dark blue ROI (area of normal prostate tissue) were used as empty background and tissue background, respectively. Green ROI (lesion 1) and pink ROI (lesion 2) show increased signal; histopathologic analysis confirmed cancer tissue in these areas. Orange ROI in B shows increased signal from area without cancer cells.
In total, 23 CLI images of the intact prostate were acquired and analyzed in the 10 patients.
Patients 1, 5, and 6 had a PSM on histopathology (Table 1). In patients 1 and 5, the elevated signal levels enabled correct identification of these margins on CLI (Table 3; Fig. 2). International Society of Urological Pathology Gleason grades (ISUP-GGs) at the resection margin were 5 in patient 1, with a diameter of 2 mm, and 4 in patient 5, with a diameter of 8 mm. Tumor radiance and TBR were 19,061 and 6.7, respectively, in patient 1 and 6,131 and 1.9, respectively, in patient 5. In patient 6, no elevated signal could be identified on CLI, and this patient had an ISUP-GG of 1 at the margin (right dorsal lobe) on histopathology, with a diameter of 3 mm.
CLI Measurements
Gray-scale photographs overlaid with Cerenkov signal for patients 1 (A), 5 (B), and 6 (C), with PSMs. Elevated signal (arrow) from tumor can be seen in A and B. Elevated signal without histopathologic tumor cell correlation is displayed in C (arrow). Immunohistochemistry showed PSMA expression of benign tissue in this region.
In total, 35 ROIs were drawn in areas displaying an elevated CLI signal: 24 in the base, 8 in the apex, and 3 in the ventral side (Table 3). However, 25 of the 35 ROIs (71.5%) were confirmed by pathology to contain tumor cells, some with margins of up to 3 mm. The median tumor radiance in these areas was 11,301 photons/s/cm2/sr (range, 3,328–25,428 photons/s/cm2/sr), and the median TBR was 4.2 (range, 2.1–11.6).
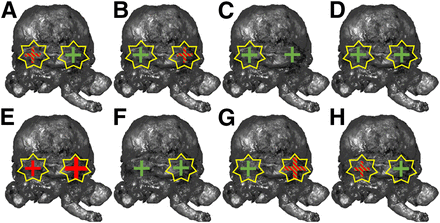
PSMA immunohistochemistry was performed on the last slices of the prostate base in the first 8 patients for whom standard histopathology suggested false-positive results, to assess PSMA expression in these areas (Fig. 3; Table 4). PSMA immunohistochemistry demonstrated a moderate to strong PSMA immunohistochemistry signal that corresponded to CLI in benign tissue in 7 of 8 patients. In 5 (62.5%) patients, underlying PC tissue with PSMA immunohistochemistry expression was also observed. All cancer areas showed an elevated CLI intensity.
PSMA immunohistochemistry correlation of prostate base, running from patient 1 (A) to patient 8 (H). Green crosses = PSMA immunohistochemistry expression of benign gland tissue; red crosses = PSMA immunohistochemistry expression of carcinoma; green and red striped crosses = PSMA immunohistochemistry expression of benign gland tissue and carcinoma. Crosses display analyzed side (left or right prostate base). Additionally, larger crosses symbolize higher amount of tumor cells. Yellow stars display increased Cerenkov intensity in this area.
PSMA Immunohistochemistry
In 4 patients, radiation safety measurements were performed and demonstrated that the intensity level in the excised specimen was below the permitted exemption levels stipulated by the German radiation protection ordinance (68Ga < 100 kBq).
DISCUSSION
This first-in-men study evaluated the feasibility of intraoperative 68Ga-PSMA CLI for assessing tumor margin status in patients with high-risk primary PC undergoing RPE and extended pelvic lymph node dissection. CLI seems to be able to successfully detect positive histopathologic resection margins with high ISUP-GGs, and all ROIs containing PSMA-immunohistochemistry–positive cancer tissue showed elevated Cerenkov intensity. The low 68Ga-PSMA activity at the time of surgery implies that the prostate specimen presents no considerable radiation burden for the CLI operator or pathologist and can be treated as nonradioactive material.
One patient had a PSM with an ISUP-GG of 1, but this margin did not display an increased signal on CLI. As these low-grade cancers are known to have a lower PSMA expression, this finding may explain false-negative CLI results (30).
To further examine Cerenkov signals, we incised the prostate gland at the main tumor lesion in 2 patients. In both patients, 2 suggestive areas were detected. Correlation of histopathology and CLI proved a detection rate of 100% in patient 2 and 50% in patient 1. In patient 1, superficial cancer cells were described on the left side. Because the main cancer nidus was at the right dorsal prostate base, we assume that the cancer tissue was overlaid through a thin layer of normal tissue and therefore could be not visualized on CLI. This assumption is supported through the higher TBR of the left ROI (2.95 vs. 2.46).
Although 68Ga-PSMA CLI of prostatectomy specimens seems feasible for assessing the status of tumor-resection margins, 10 of the 35 ROIs showed elevated signal levels without histopathologic evidence of PC tissue at the resection margin. There are several possible reasons for these false-positive findings. First, CLI intensity can emerge from PSMA expression and tracer uptake in noncancerous tissues. To analyze the prostate base, where CLI was positive despite negative histopathology findings, PSMA immunohistochemistry of the last slice of the prostate base was performed on 8 patients. In 14 of 16 (87%) areas, PSMA staining of benign tissue was observed. Eight of these may have caused a false-positive signal on CLI because they were close to the tissue’s surface. Second, the mean positron range of 68Ga is ±2.8 mm (31), and thus, Cerenkov luminescence can be produced from a few millimeters underneath the tissue’s surface. To enable more superficial imaging, a short-pass optical filter might be used in the future to exclude the longer-wavelength Cerenkov photons that have a deeper tissue penetration (i.e., the red and near-infrared Cerenkov photons). Another option would be to use an 18F-labeled PSMA tracer such as 18F-PSMA-1007 (17). As 18F positrons have a lower energy than 68Ga (0.64 vs. 1.9 MeV), the mean positron range in tissue is significantly shorter (0.54 vs. 2.83 mm), thus facilitating submillimeter margin assessment (31). Lastly, 68Ga-PSMA-11 is known to be excreted via the urinary tract, thus potentially causing elevated signals at the bladder neck or prostate base through urinary contamination (32). One method for minimizing the risk of prostate surface contamination from urine is to rinse the prostate with sodium chloride solution before CLI image acquisition. However, this step was not performed in this study. Another method would be to use a tracer that has nonrenal clearance, such as 18F-PSMA-1007 (17,33).
The preclinical study by Olde Heuvel et al. found 0.14 kBq/mL to be the minimum detectable intensity for a 300-s 8 × 8 binning acquisition (34). Because of the long interval between tracer injection and CLI in our study (∼6 h), the activity levels in the prostate at the time of CLI were low (median 68Ga-PSMA activity of 0.34 kBq/mL) and close to the limit of detection. To avoid a loss of imaging quality, a long acquisition time is essential. Nevertheless, tumor margin assessment on CLI could be performed in all 10 patients. To increase the difference in CLI signal intensity between benign and PC tissue, the time from injection to CLI can be reduced. Such a reduction may improve the detectability of PSMA cancers of low avidity, including low ISUP-GG. Alternatively, the workflow could be changed so that on the day of surgery no PET imaging is performed and only a reduced amount of tracer is injected.
Long-term prediction of PC-specific mortality is based mainly on histopathology features as described by Eggener et al.: poorly differentiated cancer, with a Gleason grade of 8–10, and seminal vesicle invasion are prime determinants of PC-specific mortality after RPE (35). Findings of Martini et al. demonstrated that only unfavorable PSMs (≥3 mm or multifocal positive margins) significantly influence the risk of metastasis in PC (36). Currently, intraoperative frozen-section analysis is the gold standard for assessing removal of all PC tissue. If the addition of short-pass optical filters reduces the penetration depth of CLI, frozen-section analysis might, in the future, be reduced to conspicuous areas only.
To assess sensitivity and specificity, a single-center clinical study is scheduled to commence in early 2020. The recruitment target is 40 patients, with the first patient expected to be recruited in quarter 1 of 2020. In these patients, the CLI protocol will be improved by implementing a short-pass optical filter. In parallel to the planned clinical study, we are performing preclinical experiments using 3-dimensionally printed prostate models to evaluate the penetration depth and resolution of CLI in correlation with tracer activity levels.
CLI uses nuclear medicine methods to inform urologic surgery for improved local treatment of PC. This new imaging modality brings the urology and nuclear medicine communities more closely together in making progress on PC (37).
CONCLUSION
Intraoperative 68Ga-PSMA CLI in PC is a promising tool to improve surgery. CLI provides high-resolution information on the whole prostate gland that may assist surgeons in assessing margin status by providing a good correlation to histopathologic examination.
DISCLOSURE
Maarten Grootendorst in an employee of Lightpoint Medical Ltd. Wolfgang P. Fendler is a consultant for Ipsen, Endocyte, and BTG and received personal fees from RadioMedix outside the submitted work. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is CLI useful for displaying PC cells near or at the surface of the prostatectomy specimen?
PERTINENT FINDINGS: In this feasibility study of 10 patients undergoing radical prostatectomy with a 68Ga-PET/CT scan on the same day, CLI was able to detect cancer cells near and at the surface of the prostate. These results are the basis for initiation of further prospective studies.
IMPLICATIONS FOR PATIENT CARE: CLI is a promising technique for intraoperative evaluation of the whole prostate surface regarding PSMs.
- © 2020 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication December 4, 2019.
- Accepted for publication February 5, 2020.