Abstract
Our objective was to use 18F-FDG PET/CT to identify a high-risk subgroup requiring therapeutic intensification among patients with locally advanced cervical cancer (LACC) and paraaortic lymph node (PALN) involvement. Methods: In this retrospective multicentric study, patients with LACC and PALN involvement concurrently treated with chemoradiotherapy and extended-field radiotherapy between 2006 and 2016 were included. A senior nuclear medicine specialist in PET for gynecologic oncology reviewed all 18F-FDG PET/CT scans. Metabolic parameters including SUVmax, metabolic tumor volume, and total lesion glycolysis (TLG) were determined for the primary tumor, pelvic lymph nodes, and PALNs. Associations between these parameters and overall survival (OS) were assessed with the Cox proportional hazards model. Results: Sixty-eight patients were enrolled in the study. Three-year OS was 55.5% (95% confidence interval, 40.8–68.0). When adjusted for age, stage, and histology, pelvic lymph node TLG, PALN TLG, and PALN SUVmax were significantly associated with OS (P < 0.005). Conclusion: 18F-FDG PET/CT was able to identify predictors of survival in the homogeneous subgroup of patients with LACC and PALN involvement, thus allowing therapeutic intensification to be proposed.
- 18F-FDG PET/CT
- locally advanced cervical cancer
- paraaortic lymph node involvement
- SUVmax
- total lesion glycolysis
The diagnosis of paraaortic lymph node (PALN) involvement in patients with locally advanced cervical cancer (LACC) is essential for risk stratification. Together with tumor volume, International Federation of Gynaecology and Obstetrics (FIGO) stage, depth of stromal cervix invasion, lymphovascular space invasion, locoregional extension, response to chemoradiotherapy, and nodal status, PALN involvement is inversely associated with survival (1–4). Since the metaanalysis of Green et al., the treatment of LACC has consisted of pelvic radiotherapy with concurrent low-dose platinum-based chemotherapy, followed by uterovaginal brachytherapy (5–10), as recommended in the Guidelines of the European Society for Medical Oncology, the National Cancer Institute, and the American Society of Clinical Oncology in 2016. Extended-field radiotherapy is therefore performed on patients whose disease has spread to the PALNs. Despite extended-field radiotherapy, patients with PALN involvement have a substantially shorter median survival (33 mo), with 40% of patients developing distant metastases (4,11,12). The use of systemic adjuvant or neoadjuvant therapies, in combination with chemoradiotherapy, may be beneficial for improving the prognosis of these patients. However, many of these treatments are still being trialed, as improvements in survival are often associated with increases in toxicity (13–15). It is therefore crucial to identify factors that predict response to treatment to improve the survival of patients exhibiting the most unfavorable prognostic features. Not surprisingly, the consensus from the Gynaecologic Cancer Intergroup concludes that there is a real need for new trials directed at these high-risk cervical cancer patients (12).
18F-FDG PET/CT is recommended as part of an extension to the LACC assessment and for detecting lymph node metastases in the paraaortic region (11,16). Several authors have also evaluated the prognostic value of 18F-FDG PET/CT metrics. Of these, the SUVmax has been the most widely studied (17). However, the populations investigated in these types of studies were mostly heterogeneous, and patients with PALN involvement were underrepresented.
The objective of this study was to assess the link between metabolic 18F-FDG PET/CT parameters and overall survival (OS) in patients with LACC specifically having PALN involvement.
MATERIALS AND METHODS
Patients and Treatment
Patients with LACC (FIGO stage IB2–IVA) and positive PALNs, treated at the Claudius Regaud Cancer Centre, at the University Hospital of Toulouse, and at the Paoli-Calmettes Cancer Centre, Marseille, France, between January 2006 and November 2016, were selected for this study. All patients had cervical cancer confirmed by biopsy and underwent a physical examination, an initial pelvic MRI scan, and an 18F-FDG PET/CT scan. Cervical cancers were retrospectively classified with the 2009 FIGO staging system. The diagnosis of PALN spread was based on the initial 18F-FDG PET/CT scan or on histopathologic examination of harvested PALNs as part of lymphadenectomy staging. Micrometastatic PALN involvement was defined as a PALN metastasis of 0.2–2 mm (18).
Patients were treated with pelvic and paraaortic external radiotherapy in 25 fractions of 1.8 Gy for a total dose of 45 Gy for 5 wk with concomitant platinum-based chemotherapy (cisplatin at a dose of 40 mg/m2 per week or carboplatin [area under the curve] when cisplatin was poorly tolerated). Gynecologic examination and 1.5-T MRI with gadolinium were performed during the first week after completion of the 45-Gy radiotherapy. Treatment varied, depending on the type of response detected by MRI. Pretherapeutic tumor size was defined as the largest tumor dimension measured on pretherapeutic MRI. Tumor size after therapy was the largest tumor size measured after 45-Gy radiotherapy. Response was considered good when the maximum diameter of the tumor decreased by more than 50% at the 45-Gy MRI evaluation. When the decrease was more than 50%, further treatment consisted of additional pulsed dose-rate intracavitary brachytherapy for an equivalent total dose of 80–90 Gy. Before 2008, additional boosts of up to 65 Gy in total were sometimes given at the end of brachytherapy in external radiotherapy in cases of macroscopic pelvic or PALN or parametrial involvement. When intensity-modulated radiation therapy became available, a simultaneous integrated boost was given on metastatic pelvic and PALN involvement at doses of 57.5 Gy in 25 fractions. If the tumor reduction rate was less than 50%, completion treatment was left at the discretion of the treating physician and included either brachytherapy alone or completion surgery to resect any residual tumor with clearly defined margins. This surgery was mostly performed 6 wk after preoperative brachytherapy for an equivalent total dose of 60 Gy. For patients recruited at the Paoli Calmette Institute, systematic completion surgery was performed 6 wk after preoperative brachytherapy, regardless of whether the response was complete.
We included 4 patients for whom distal metastatic disease was identified at the initial diagnosis, was subsequently treated with neoadjuvant chemotherapy, and produced a good tumor response, allowing evaluation of a complete curative treatment regimen, which included extended paraaortic irradiation fields.
Follow-up included a clinical examination every 4 mo for 2 y and every 6 mo for the following 3 y. Imaging (MRI, CT, PET/CT) complementary to the clinical examination was performed as part of the follow-up. Relapse was defined as the emergence of a tumor locus after a complete response to treatment based on end-of-treatment reassessment imaging examinations (MRI or PET/CT). Progression was defined as an increase in the number or size of tumor foci under treatment, or after treatment in the event of a partial response, based on the evaluation of imaging according to PERCIST (or RECIST when PET/CT was not available).
Exclusion criteria were the following: rare histologic subtypes, a lack of available 18F-FDG PET/CT images for centralized analysis by the 2 independent investigators, the absence of PALN involvement, peritoneal carcinosis, patients whose treatment was not given with curative intent, and a lack of extended-field radiotherapy for PALN involvement.
The study was approved by the local institutional review board from both institutions, and all patients gave written informed consent to use their data.
18F-FDG PET/CT Parameters and Interpretation
All patients underwent pretreatment 18F-FDG PET/CT scans. The PET data were reconstructed using an iterative fully 3-dimensional algorithm, with the CT images being used for attenuation correction, anatomic identification, and localization of radiotracer uptake.
18F-FDG PET/CT imaging based on a standardized protocol was performed as part of the initial work-up before administration of any treatment. Whole-body images were obtained using a full-ring PET/CT scanner. All patients fasted for at least 6 h before injection of the 18F-FDG. Blood glucose levels were checked before the injection, and the injected dose and the time between injection and acquisition were recorded. Patients underwent full-body scans after a resting period of 60 ± 6 min. The total scan duration varied with the type of PET/CT camera used, the 18F-FDG injected dose, and the number of bed positions, and the protocols were adapted to each system’s count rate. Because of bladder refilling, complementary pelvic acquisitions were sometimes performed after intravenous administration of furosemide (20 mg).
The PET/CT scanners used for this study were a Siemens Biograph 6, a GE Healthcare Discovery IQ, a GE Healthcare DST4, and a Philips Gemini TF16. A senior nuclear medicine specialist in PET for gynecologic oncology reviewed all 18F-FDG PET/CT scans. All patient metabolic parameters were reviewed by another independent senior nuclear medicine specialist using a double-blind approach, to ensure homogeneous measurement of metabolic variables throughout the study. Segmentation of tumor volumes for cervical tumors, as well as pelvic lymph nodes and PALNs, was performed using the GE Healthcare software AWServer 3.0, with an automatic threshold set at 40% of SUVmax, in accordance with the European Association of Nuclear Medicine guidelines (19). This automatic contouring was manually corrected if necessary, taking CT and MRI data into account.
Metabolic parameters included SUVmax, SUVmean, metabolic tumor volume (MTV), and total lesion glycolysis (TLG) measurements of the primary cervical tumor, pelvic lymph nodes, and PALNs, with data from the most active site used in the subsequent analyses. The size of the primary cervical tumor was determined from the CT image along 2 axes of the transverse plane. The SUVmax was defined as the maximum uptake value of tracer within a tumor lesion. The TLG expresses the whole glycolysis of a lesion. The TLG was obtained as the product of MTV and SUVmean.
Statistical Analysis
We evaluated any potential associations between OS and putative prognostic factors with the Cox proportional hazards model. To meet the conditions for applying the model while maintaining sufficient power for the analysis, quantitative variables were dichotomized at the median, with the exception of the PALN SUVmax, for which the threshold of 3.3 proposed by Yen et al. (20) was used. We evaluated all potential associations between individual metabolic parameters and survival by bivariate analysis and, subsequently, by models adjusted for specific clinical factors (i.e., age, histologic subtype, and FIGO stage). P values of less than 0.05 were considered significant. We present results for parameters whose P values were less than 0.20 in adjusted models. A dose effect was also explored graphically using a Cox survival curve derived from terciles of each variable. Statistical analyses were performed using STATA release 14.2 (StataCorp LP).
RESULTS
Sixty-eight patients were included in the study. Their characteristics are summarized in Table 1. Forty-two patients (66.7%) had pretherapeutic surgical paraaortic staging by laparoscopy. Four patients (9.5%) had micrometastatic PALN involvement.
Patient Characteristics
Eleven of the 68 patients did not receive any brachytherapy: 2 because of vaginism, 1 because of neutropenia, 1 because of progression during the course of treatment, and 7 because of noncompliance with the procedure.
Before chemoradiotherapy, the median cervical tumor size was 57.5 mm (range, 26–127 mm). Sixty-two patients had abnormal pelvic lymph nodes, and the median number of positive pelvic lymph nodes was 4 (range, 1–12). Fifty-two patients had abnormal PALNs, and the median number of positive PALNs was 1 (range, 1–8). Metabolic parameters before 18F-FDG PET/CT treatment are presented in Table 2.
Metabolic Parameters Before Treatment
After a median follow-up of 24.3 mo (95% confidence interval [CI], 3.5–138.7 mo), 31 patients (45.6%) had succumbed to their disease. Sixteen patients (23.5%) had progressed, and 21 patients (39.6%) had relapsed. Metastases developed in 62.5% and 90.5% of the patients who progressed or relapsed, respectively. OS was 39.7% (95% CI, 56.5–79.7) after 2 y and 55.5% (95% CI, 40.8–68.0) after 3 y. Median OS was 43.9 mo (Fig. 1).
Kaplan–Meier plots of overall survival.
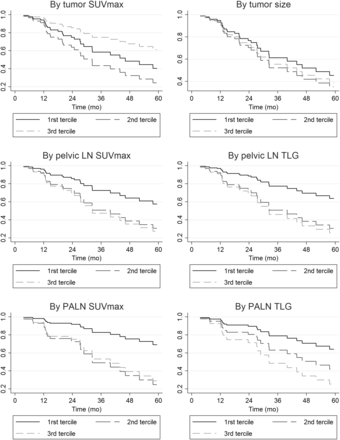
In terms of patient characteristics, stage IIIB or IV tumor was associated with a significantly poorer outcome (hazard ratio [HR], 4.08; P = 0.021) (Table 3). Among the 18F-FDG PET/CT pretreatment parameters (Table 4), adjusted for patient age, stage, and histology, pelvic lymph node TLG, PALN TLG, and PALN SUVmax were significantly associated with survival (P < 0.005). Patients with a higher cervical tumor SUVmax or a higher pelvic lymph node SUVmax also tended to have a higher mortality rate, at the limit of significance (P = 0.076 and P = 0.077, respectively). Graphically (Fig. 2), we observed a dose effect for pelvic lymph node and PALN SUVmax and for pelvic lymph node and PALN TLG.
OS According to Age, Histologic Subtype, and FIGO Stage (Cox Models)
OS According to Metabolic Parameters (Cox Models)
Cox analysis survival curves by terciles of metabolic parameters. LN = lymph node.
DISCUSSION
Despite current chemoradiotherapy management with extended-field radiotherapy, the prognosis of LACC patients with PALN involvement remains poor (2,12,21,22). Overall, the results from our series are consistent with data reported in the literature. OS was 39.7% (95% CI, 56.5–79.7) after 2 y and 55.5% (95% CI, 40.8–68.0) after 3 y. Sixteen patients in our study (23.5%) progressed, and 21 patients (39.6%) relapsed. Metastases developed in 62.5% and 90.5% of the patients who progressed or relapsed, respectively. The rate of metastatic relapse raises questions about the presence of occult metastases at the initial diagnosis. Systemic therapies in addition to standard chemoradiotherapy may prove beneficial in these patients. The phase III INTERLACE (NCT01566240) and OUTBACK (NCT01414608) trials are currently under way to evaluate these approaches by analyzing the potential benefits of carboplatin and paclitaxel chemotherapy for neoadjuvant or adjuvant applications, respectively. Regrettably, the presence of lymph node involvement above the common iliac artery is an exclusion criterion for these trials. Moreover, the significant side effects of these treatments compel research teams to select subgroups of patients at high risk of relapse using novel prognostic factors.
In addition to its benefits in LACC staging, 18F-FDG PET/CT could therefore also provide useful prognostic data in these high-risk patient groups. To our knowledge, our current study is not only the first but also the largest to evaluate the predictive power of 18F-FDG PET/CT in an LACC patient population with PALN involvement. It shows that in patients matched for age, stage, and histology, pelvic lymph node TLG (HR, 3.72; P = 0.05), PALN TLG (HR, 3.11; P = 0.039), and PALN SUVmax (HR, 4.80; P = 0.011) are independently associated with OS. TLG is the product of MTV and the SUVmean of the tumor lesion and is related to the size and metabolism of the lesion. TLG also reflects the activity of the entire lesion volume, unlike SUVmax, which is a maximum value retained from the voxel that fixes most metabolites. In addition, TLG is directly dependent on the extent of tumor volume included at the site.
Pelvic lymph node SUVmax was associated with OS in univariate analysis (HR, 2.27; P = 0.043). The prognostic significance of lymph node SUVmax in cervical cancer was highlighted by Kidd et al. in a prospective study of 83 patients (FIGO IB1–IIIB). They demonstrated that a high pelvic lymph node SUVmax was associated with persistence of the disease after treatment (P = 0.0025), with relapse (P = 0.0035), and with a worse OS (P = 0.0378) (23). For PALN SUVmax, a study published by Yen et al. involving 70 patients (FIGO I–IV), of whom 20 had PALN involvement, identified a PALN SUVmax above a 3.3 threshold to be significantly related to poor OS (20). The same threshold used in our study revealed a significant association between PALN SUVmax and OS in univariate analysis (HR, 4.67; P = 0.05) and also after adjusting for age, FIGO stage, and histologic subtype (HR, 4.8; P = 0.011).
In a retrospective study of 56 patients (FIGO IIB–IIIB), Chong et al. found that node SUVmax, node MTV, and node TLG correlated with relapse-free survival in univariate analysis, but only node SUVmax was shown to predict relapse-free survival in multivariate analysis (24). Unlike our study, in which the highest node MTV and TLG metabolic values were those of the pelvic lymph nodes or PALNs, the values of node MTV and TLG were defined by Chong et al. as the sum of the MTV and TLG values of each hypermetabolic node. In any case, data reported in the literature should be interpreted conservatively in light of the great variability due not only to the different characteristics of cohorts but also to the different measurement criteria used.
The SUVmax for the primary cervical tumor is the most commonly used metabolic parameter in current routine practice, and its prognostic benefit in the management of cervical cancers has been extensively described in the literature (17,25–28). The prognostic value of primary cervical tumor MTV and TLG has also been investigated in several studies (29–33). In our study, none of the metabolic parameters of primary cervical tumors appeared to be correlated with OS. Unlike the populations in previous studies, our study population was highly specific since it consisted exclusively of LACC with PALN involvement, which is well known for its dismal outcome. The other studies examined very heterogeneous groups of patients with regard to the stage of the primary disease and therefore covered a much broader spectrum with very different prognoses.
18F-FDG PET/CT is also a useful test for evaluating metabolic tumor response at the end of treatment. Several studies have shown that the metabolic response determined by 18F-FDG PET/CT, performed on average 3 mo after the end of radiotherapy, is predictive of survival (33–36). However, the analysis of the metabolic response by 18F-FDG PET/CT is very much operator-dependent, because of the application of visual scales and criteria that are sometimes complex to use routinely (PERCIST (37)), as well as the prerequisite for specific anatomic understanding of pelvic oncology imaging practices. The measurement of TLG in a residual cervical tumor using posttherapeutic 18F-FDG PET/CT might provide functional information in addition to the morphologic information provided by the reevaluation of MR images. In addition, by determining thresholds of 18F-FDG PET/CT reassessment of metabolic parameters, the evaluation of tumor responsiveness might be standardized. Specifically, the analysis of tumor responses with TLG might be developed with an artificial intelligence approach using automatic segmentation and software learning, that is, deep learning. As such, it might constitute a useful aid in interpreting results and even play an important role in centers not staffed with nuclear medicine, gynecology, or oncology specialists.
Our study had some limitations. It was retrospective and small, as can be justified by the choice of the patient subgroup. It included patients from 2 cancer control centers, thus introducing a degree of heterogeneity in the therapeutic management. Nevertheless, 93% of patients were treated with a complete chemoradiotherapy schedule, and all received extended-field radiotherapy to the paraaortic area. Despite the established recommendations, this heterogeneity of management has been previously highlighted in the literature in this subgroup of patients (38). The 18F-FDG PET/CT data from our study are based on the technical specifications of several different PET/CT camera manufacturers, who in some cases use very different image acquisition and reconstruction methods. To limit this heterogeneity, we opted to eliminate data from machines that were too old, and not to use the most recent image reconstruction algorithms, which are known to modify significantly the way SUVmax is calculated (QClear; GE Healthcare). In addition, the centralized review of all 18F-FDG PET/CT examinations by a nuclear medicine specialist ensured a more accurate comparison of 18F-FDG PET/CT metabolic parameters.
CONCLUSION
18F-FDG PET/CT helped to identify predictors of survival in the homogeneous subgroup of patients with LACC and PALN involvement. Further studies are necessary to validate the prognostic impact of PALN SUVmax and PALN and pelvic lymph node TLG as new metabolic parameters in this high-risk subgroup, especially to allow therapeutic intensification to be adopted.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Can 18F-FDG PET/CT help identify predictors of survival in patients with LACC and PALN involvement to allow intensification of treatment?
PERTINENT FINDINGS: Pelvic lymph node TLG, PALN TLG, and PALN SUVmax were significantly associated with OS (P < 0.005).
IMPLICATIONS FOR PATIENT CARE: 18F-FDG PET/CT was able to identify predictors of survival in the subgroup of patients with LACC and PALN involvement, thus allowing therapeutic intensification to be proposed.
Acknowledgments
We thank our methodologist, Samuel Ladias, for his contribution.
Footnotes
Published online Feb. 7, 2020.
- © 2020 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication October 25, 2019.
- Accepted for publication January 28, 2020.