Abstract
Reliable standards and criteria for somatostatin receptor (SSTR) PET are still lacking. We herein propose a structured reporting system on a 5-point scale for SSTR PET imaging, titled SSTR-RADS version 1.0, which might serve as a standardized assessment for both diagnosis and treatment planning in neuroendocrine tumors. SSTR-RADS could guide the imaging specialist in interpreting SSTR PET scans, facilitate communication with the referring clinician so that appropriate workup for equivocal findings is pursued, and serve as a reliable tool for patient selection for planned peptide receptor radionuclide therapy.
- 68Ga-DOTATATE/-TOC
- SSTR
- somatostatin receptor
- PET
- PRRT
- peptide receptor radionuclide therapy
- neuroendocrine tumor
- RADS
Because of the promising results of recent randomized clinical trials, peptide receptor radionuclide therapy (PRRT) of inoperable, metastasized neuroendocrine tumors (NETs) is increasingly being used (1,2). As a prerequisite for endoradiotherapy using hot somatostatin analogs (SSAs), somatostatin receptor (SSTR) PET using agonists including 68Ga-DOTATOC/DOTATATE and 68Ga-DOTA,1-Nal3-octreotide (68Ga-DOTANOC) or antagonists such as 68Ga-NODAGA-JR11 (68Ga-OPS202) must demonstrate sufficient radiotracer uptake in suggestive NET lesions (3–5). Hence, from a long-term perspective, increased use of SSTR PET can be envisaged. However, in contrast to the clinically well-established 4-point Krenning scale in terms of SSTR scintigraphy (111In-pentetreotide; OctreoScan), which is mainly based on liver-versus-tumor uptake (6), reliable standards and criteria for SSTR PET are still lacking (7). Of note, numerous studies have reported on pitfalls in interpreting SSTR PET scans: the physiologic distribution includes tissues that potentially exhibit SSTR, such as the pituitary gland, tonsils, thyroid, adrenal glands, liver, spleen, or the head of the pancreas (8,9); macrophages also express SSTR on the cell surface, and increased inflammatory activity might lead to potential false-positive radiotracer uptake (10,11); individual tumors can have heterogeneous levels of SSTR expression related to their degree of differentiation (G1–G3) (7); and short- and long-acting SSA and chemotherapeutic agents could influence PET imaging due to receptor saturation and fluctuations of SSTR expression on the tumor cell surface (3,11,12).
Hence, in analogy to reporting and data systems that have been described for multiple different organs (13–16) as well as for molecular imaging of prostate cancer (prostate-specific membrane antigen [PSMA]-reporting and data systems [RADS]) (17), we herein propose a structured reporting system on a 5-point scale for SSTR PET imaging, titled SSTR-RADS version 1.0, which might serve as a standardized assessment for both diagnosis and treatment planning in NET. SSTR-RADS could guide the imaging specialist in interpreting SSTR PET scans, aid communication with the referring clinician so that appropriate workup for equivocal findings is pursued, and serve as a reliable tool for patient selection for planned PRRT.
PATIENT POPULATION
Because the herein presented data comprise a retrospective analysis of routinely acquired data, the local ethic committee waived the need for further approval. All patients gave written and informed consent to the procedures, and all patients provided written informed consent for scientific analysis of the obtained data.
OVERVIEW OF SSTR-RADS AND DESCRIPTION OF DIFFERENT CATEGORIES
Table 1 gives an overview of the SSTR-RADS in its current form, version 1.0. The reporting system is mainly based on the format of the recently published PSMA-RADS version 1.0 (17), consensus findings from the clinical experience of the 2 involved centers (Johns Hopkins University School of Medicine and European Neuroendocrine Tumor Society–Center of Excellence Würzburg University), as well as an extensive search in electronic databases including PubMed (www.ncbi.nlm.nih.gov), Science Direct (www.sciencedirect.com), MEDLINE (https://www.nlm.nih.gov/bsd/pmresources.html), and Library of Congress (https://www.loc.gov). The search strategy included the following key words: “SSTR-PET” or “PRRT” or “DOTATATE” or “DOTATOC” and “Standardization”, without restrictions to language or publication date. When the SSTR-RADS reporting system is used, multiple different goals might be achieved: reflect the level of confidence of an imaging reader on the presence of a NET tumor lesion, provide guidance as to which lesions should be considered physiologic rather than pathologic, which test might be appropriate as a next step in the diagnostic algorithm (based on the SSTR PET findings), and the establishment of which patients might be suitable candidates for subsequent PRRT. Table 2 summarizes the uptake level on SSTR PET using a 3-point scale, which goes from score 1 (focal uptake, but lower than/equal to blood pool) through score 2 (higher than blood pool, but lower than/equal to physiologic liver uptake) to score 3 (higher than physiologic liver uptake). These categories will subsequently be referred to as “level 1,” “level 2,” or “level 3” uptake, respectively.
Overview of SSTR-RADS 1–5
A 3-Point Qualitative Assessment Scoring for Defining Uptake Level in an SSTR-Avid Lesion on a SSTR PET Scan
SSTR-RADS-1
SSTR-RADS-1 signifies definitively benign lesions, as known by previous biopsy or pathognomonic anatomic imaging. These lesions are further divided into SSTR-RADS-1A and SSTR-RADS-1B (Table 1). SSTR-RADS-1A indicates definitively benign findings without any abnormal uptake (normal physiologic biodistribution of an SSTR imaging agent (Fig. 1) (18,19). On the other hand, SSTR-RADS-1B includes those lesions, which although benign, demonstrate discernable radiotracer uptake, for example, prostatitis, benign prostatic hyperplasia (Fig. 2), or intense uptake in the thyroid (e.g., due to a known thyroid adenoma, Supplemental Fig. 1 [supplemental materials are available at http://jnm.snmjournals.org], level 2–3) which has been previously confirmed by biopsy or definitive diagnostic imaging (20). Of note, it has to be emphasized that SSTR-RADS scores can be dynamic based on the workup that the patient has had done: If an intensively avid thyroid nodule is primarily classified as SSTR-RADS-3C (no previous imaging or other work-up), then biopsied and found to be an adenoma, this finding would be rescored as SSTR-RADS-1B.
SSTR-RADS-1A. Coronal 68Ga-DOTATOC PET whole-body maximum-intensity projection (A) demonstrating physiologic tracer distribution. No sites of abnormal uptake can be appreciated. Normal biodistribution of agent is seen, including uptake in pituitary gland, thyroid, adrenal glands, bowel, liver, and spleen (18,19). Radiotracer is excreted via urinary tract. Arrow indicates physiologic finding in uncinate process, which is also demonstrated by axial 68Ga-DOTATOC PET (B), axial CT (C), and axial 68Ga-DOTATOC PET/CT (D).
SSTR-RADS-1B. Image of patient with increased Chromogranin A levels, referred for initial staging. Axial 68Ga-DOTATOC PET (A), axial CT (B), and axial 68Ga-DOTATOC PET/CT (C) demonstrating increased uptake in prostate (arrow), for example, caused by prostatitis or due to benign prostatic hyperplasia.
SSTR-RADS-1B might help the reader to describe a region of increased uptake but without raising a concern of malignancy (17). PRRT is definitely not considered.
SSTR-RADS-2
The SSTR-RADS-2 category describes lesions with uptake (level 1) in soft-tissue sites atypical for metastatic NET (e.g., axillary lymph nodes) or uptake in bone lesions atypical for NET (e.g., strongly suggestive of being degenerative in nature, Fig. 3). In brief, SSTR-RADS-2 includes those lesions with low levels of SSTR expression or nonspecific radiotracer uptake and that are atypical sites for NET lesions. SSTR-RADS-2 lesions are almost certainly benign; PRRT is definitely not considered.
SSTR-RADS-2. Likely benign skeletal finding with uptake in patient with NET of pancreatic origin (G1, Ki-67 = 2%). Axial CT (A), axial 68Ga-DOTATOC PET (B), axial 68Ga-DOTATOC PET/CT (C), and coronal CT (D) showing lytic-appearing lesion involving inferior endplate of lumbar vertebral body (arrow). Strongly suspected to be degenerative (a Schmorl’s node), this intravertebral disk herniation would be classified as SSTR-RADS-2.
SSTR-RADS-3
SSTR-RADS-3 includes those imaging findings that require further workup (biopsy, if sampling is possible, or follow-up imaging). Many of these lesions are suggestive of, but not definitive for, NET tumors. SSTR-RADS-3A signifies level 1–2 uptake in soft-tissue sites typical for NET metastases, such as in regional lymph nodes (Fig. 4), whereas SSTR-RADS-3B refers to level 1–2 uptake in bone lesions not atypical for NET (Fig. 5). In both cases, initial follow-up imaging (SSTR PET or whole-body MRI) might be necessary to confirm definitive diagnosis, although the final interpretation may also depend on Ki-67/grading (21). Similar to PSMA-RADS, we would recommend follow-up imaging after 3–6 mo (3,22); any progression of the lesions would lead to an upgrade to either SSTR-RADS-4 or SSTR-RADS-5 (17). However, the Ki-67 index/histopathologic grading has to be considered in the context of NET; in the case of an increased proliferation index, follow-up imaging after 3 mo would be preferred. For SSTR-RADS-3B, the treatment algorithm should depend on the biology and number of positive lesions: Single positive lesions might rather be followed or treated with a locoregional procedure, whereas an increased number of growing, SSTR-expressing metastases might guide the treating physician to consider PRRT (3,23).
SSTR-RADS-3A. Low-level uptake in mesenteric lymph node in midabdomen of patient diagnosed with ileal NET (G1, Ki-67 = 2%). Axial 68Ga-DOTATOC PET (A), axial CT (B), and axial 68Ga-DOTATOC PET/CT (C) show small (short-axis diameter, <0.5 cm) mesenteric lymph node (arrow). Degree of focal uptake was above blood pool but lower than liver (not shown), and follow-up imaging (after 3 mo) was recommended. Depending on local practice pattern, biopsy might be considered (although biopsy of this site is difficult).
SSTR-RADS-3B. Moderate uptake in bone lesion in patient with small bowel NET (G2). Axial 68Ga-DOTATOC PET (A), axial CT (B), and axial 68Ga-DOTATOC PET/CT (C) show radiotracer uptake in right fifth rib (arrow). Because of CT findings along with moderate uptake on PET, follow-up imaging was recommended.
SSTR-RADS-3C suggests another non-NET malignant process and involves intense uptake (level 3) in a site highly atypical for a NET lesion, for example, radiotracer uptake in the breast (Fig. 6) (20). In many cases, histologic diagnosis (if feasible) can help guide the selection of therapeutic options, as necessary.
SSTR-RADS-3C. Patient with right invasive, lobular breast cancer (pT3, N1, M1 (liver)). Axial 68Ga-DOTATOC PET (A), axial CT (B), and axial 68Ga-DOTATOC PET/CT (C) demonstrating intense uptake in remaining right breast (a site highly atypical for NET lesion) (arrow, level 3).
SSTR-RADS-3D lesions have a high likelihood for a malignancy (e.g., dedifferentiated NET or other malignant process), but they are negative on an SSTR PET scan. In other words, anatomic findings highly suggestive of being malignant, but demonstrating no SSTR-targeted radiotracer uptake, would be categorized as SSTR-RADS-3D. A common clinical scenario would be dedifferentiation of single NET liver lesions (in a patient with known G2 disease) demonstrating distinct heterogeneous characteristics on a subsequent 18F-FDG PET scan (SSTR PET–negative, but 18F-FDG PET–positive) (24). Different uptake patterns on 68Ga-DOTATATE and 18F-FDG scans normally indicate more aggressive disease (Fig. 7) (6,25). Hence, in terms of NET lesions suggestive of dedifferentiation, 18F-FDG PET could be performed (11,26,27). Tissue biopsy to confirm the diagnosis and to gain additional prognostic information about the non–SSTR-expressing site of disease may be of value in certain circumstances. PRRT might not be considered, but this depends on grading, overall tumor burden, kidney and bone marrow function, and overall SSTR expression on a whole-patient level. An example would be a G2 NET with most lesions demonstrating SSTR expression, but a single dedifferentiated SSTR-negative, 18F-FDG–positive lesion. A combined treatment of PRRT together with a locoregional procedure, such as selective internal radiotherapy or transarterial chemoembolization, could be performed (28,29). In some cases, SSTR-RADS-3D lesions are indicative of a non-NET malignant process (i.e., a coexisting second primary tumor with or without metastatic disease). If this is suspected, biopsy to establish the identity of the potential second malignancy is crucial to guide further imaging workup and therapy.
SSTR-RADS-3D. Non–radiotracer-avid liver lesion in patient with G2 NET of pancreatic origin with history of cold and hot somatostatin analog treatment (2 cycles of PRRT; cumulative activity, 15.4 GBq of 177Lu-DOTATOC). Axial CT (A), axial 68Ga-DOTATOC PET (B), and axial 68Ga-DOTATOC PET/CT (C) show a 2.8-cm hepatic metastasis with negligible uptake above liver background (arrow). 18F-FDG PET was recommended to assess underlying intratumoral heterogeneity/dedifferentiation, with eventual need for PET-guided biopsy being likely.
SSTR-RADS-4
SSTR-RADS-4 describes those findings having intense uptake in sites typical for NET lesions, but without definitive findings on conventional imaging. An example would be intense uptake in a locoregional lymph node (level 3), but without confirmatory findings on anatomic imaging (i.e., small lymph node or marrow-based bone lesions, which are nonsuggestive on a CT scan, Fig. 8). Given the high sensitivity and specificity of SSTR-targeted radiotracers, these lesions are likely to represent sites of NET. In brief, the main difference between SSTR-RADS-4 and SSTR-RADS-5 is the lack of anatomic correlate for SSTR-RADS-4 lesions, adding a slight uncertainty to the diagnosis of a NET lesion. Further confirmation by biopsy is generally not necessary unless prognosis or other precision medicine metrics can be gained by analyzing a tissue specimen. That being said, many of these lesions classified as SSTR-RADS-4 would be difficult to biopsy because of small size (e.g., an intensively avid small lymph node that would be difficult to target on CT guidance). On the assumption of a typical distribution of NET, PRRT can be considered (3,23).
SSTR-RADS-4. Patient with ileocecal NET (Ki-67 2%, G1), with radiotracer-avid lymph node in lower left abdomen that is too small to consider definitively disease-involved on conventional imaging. Axial 68Ga-DOTATOC PET (A), axial CT (B), and axial 68Ga-DOTATOC PET/CT (C) images show degree of uptake consistent with metastatic NET lesion (arrow, level 3). However, because short-axis diameter of lymph node was 0.6 cm (i.e., <1.0 cm), this node would generally not be considered pathologically enlarged on CT.
SSTR-RADS-5
SSTR-RADS-5 lesions show intense uptake (level 3) in sites typical for NET and with corresponding findings on conventional imaging (e.g., a liver lesion demonstrating SSTR uptake and typical appearance on CT, Fig. 9). The likelihood of a misdiagnosis is low; hence, similar to PSMA-RADS-5 or BI-RADS-5, biopsy failing to yield a definitive diagnosis has a high risk of being false-negative (15,17). As such, in these lesions, biopsy is unlikely to be of value in confirming the diagnosis, but again may be useful in certain circumstances as described in the SSTR-RADS-4 section. PRRT can definitely be considered (3,23).
SSTR-RADS-5. Image of patient with extensive SSTR-positive liver lesions. Axial CT (A and B), axial 68Ga-DOTATOC PET (C), and axial 68Ga-DOTATOC PET/CT (D) clearly demonstrating 2 intrahepatic lesions with intense radiotracer uptake (level 3) and corresponding findings on CT (arrow). This scan would be categorized as SSTR-RADS-5. PRRT could definitely be considered.
DISCUSSION
In this article, we aimed to establish a standardized reporting system for the clinically most relevant SSTR imaging agents, namely SSTR-RADS version 1.0.
In analogy to PSMA-RADS, reporting on a SSTR PET scan requires a minimum of clinical and imaging acquisition information, which should be mentioned in the clinical report (Supplemental Table 1). If fewer than/equal to 5 positive lesions can be identified, all of these sites should be classified according to the above-mentioned SSTR-RADS classification (along with an anatomic description, maximum diameter for measurable lesions, and expressed mean/maximum SUV) (3,11,17). The impression could state the SSTR-RADS scores for those lesions, but also provide an overall SSTR-RADS score. An overall SSTR-RADS of 3 without mentioning an anatomic description of increased uptake is insufficient, as further workup cannot be undertaken because the exact site of abnormal SSTR expression is still lacking (17). If more than 5 positive lesions can be detected, a dominant, representative lesion per “system” should be chosen (i.e., the largest or hottest lesion, such as primary tumor or hottest lymph node/organ metastasis); however, contrary to 18F-FDG imaging findings in NET malignancies (30), the role of a dominant/hottest lesion on a SSTR PET has not yet been fully investigated. Nevertheless, the “highest” SSTR-RADS lesion will also designate the overall PET score and might “overrule” “lower” lesions, that is, if 1 lesion is classified as SSTR-RADS-3A or -3B, but the remaining lesions are SSTR-RADS-4 or -5, the findings for SSTR-RADS-3A or -3B lesions can be waived (17). If the overall score is SSTR-RADS-4 or SSTR-RADS-5, PRRT should be considered (Fig. 10) (3,23). Nevertheless, SSTR-RADS-3C and -3D are important categories: SSTR-RADS-3C may trigger immediate further workup, whereas in SSTR-RADS-3D further workup is also required but PRRT might be more widely applicable: if only 1 single dedifferentiated lesion is present, a combined treatment of peptide-based radiotherapy with locoregional procedure might be an option. However, a high tumor burden with several dedifferentiated lesions rules out PRRT (e.g., several SSTR-negative/18F-FDG–positive liver metastases or several liver metastases that are negative on SSTR PET but are present on MRI) (11). Tumor heterogeneity (SSTR-negative/18F-FDG–positive lesions) should be reported, and local treatment of SSTR nonavid lesions should be considered (28,29).
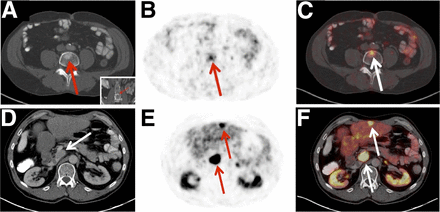
Defining overall SSTR-RADS score. Example of patient with colorectal NET (Ki-67 < 2%, G1 NET). Axial/coronal (insert) CT (A), axial 68Ga-DOTATOC PET (B), and axial 68Ga-DOTATOC PET/CT (C) reveal equivocal tracer uptake in degenerative, intravertebral disk herniation (most likely a Schmorl’s node, SSTR-RADS-2, arrow). However, axial 68Ga-DOTATOC PET (E) and axial 68Ga-DOTATOC PET/CT (F) clearly demonstrate intense uptake in liver lesion in segment III, which cannot be detected on axial CT (D) (SSTR-RADS-4), highly consistent with liver metastasis. Additionally, intense uptake in pathologically enlarged lymph node close to hilum of liver (arrow) can also be identified on both imaging modalities. As latter would be classified as SSTR-RADS-5, “highest” SSTR-RADS lesion will also designate overall PET score (i.e., SSTR-RADS-5 in this case, “overruling” other lesions). PRRT could be considered.
Despite the aforementioned goal of increasing the reader’s confidence of evaluating SSTR PET scans, the herein proposed SSTR-RADS system could be potentially applied in numerous clinical settings. Recent reports have investigated the pretherapeutic uptake of SSTR PET (SUVs) for outcome prediction in patients scheduled for PRRT, using either 177Lu- or 90Y-DOTATATE/-TOC (31–33). However, reliable outcome predictors are still intensively sought: the Delphic Consensus Assessment for GEP-NET disease management reported on the limitations of Chromogranin A alterations as well as Ki-67 for identifying potential PRRT candidates (34). Moreover, SSTR-RADS could also be expanded to other SSTR-expressing, non-GEP tumors, such as small cell lung cancer or cancer of unknown primary (35–37). Hence, in light of the increased use of SSTR PET outside of controlled clinical trials, SSTR-RADS could serve as a more sophisticated approach for outcome prediction in PRRT.
While adapting some aspects of the PSMA-RADS system to SSTR molecular imaging, we maintained the initial proposed 5-point RADS scale for prostate molecular imaging: once the underlying framework of PSMA-RADS has been successfully understood (17), it can now be readily applied for SSTR PET using SSTR-RADS, and imaging interpreters who are familiar with one system should be able to learn the other system. Nonetheless, the differences in the underlying biologies of both tumor entities have to be considered while reporting on either PSMA or SSTR PET scans. However, further analysis of interreader agreement is of utmost importance before SSTR-RADS can be implemented in clinical routine.
CONCLUSION
We herein propose a novel standardization system for SSTR PET scans, which may have utility in identifying potential pitfalls in interpretation and measure the reader’s confidence in the presence or SSTR-expressing tumor. This system may also guide the treating physician in selecting PRRT candidates. Further confirmatory work is needed to validate this proposed reporting system. Apart from that, we hope that the vision of SSTR-RADS as a way toward standardization will be pursued in daily clinical routine. SSTR-RADS is subject to continuous development and therefore, we highly appreciate any further input by imaging specialists to improve this scoring system.
DISCLOSURE
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 701983. No other potential conflict of interest relevant to this article was reported.
Footnotes
Published online Mar. 23, 2018.
- © 2018 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication December 5, 2017.
- Accepted for publication March 14, 2018.