Abstract
The purpose of this study was to evaluate 18F-FDG PET/CT for the diagnosis, management, and treatment of Erdheim–Chester disease (ECD). Methods: Our institutional database (2007–2017) was retrospectively reviewed for patients with pathologically proven ECD. A chart review yielded demographics, clinical information, and 5 categories of clinical impact. Two radiologists in consensus interpreted the images. Imaging findings were correlated with clinical data. Results: Seventy-one 18F-FDG PET/CT examinations were performed for 32 patients. The average SUVmax of the most active disease site was 9.2 (SD, 6.1). The most common sites involved were the skeleton (90.6% of patients, including 47% with axial and pelvic skeletal involvement), kidneys (81.3%), and central nervous system (CNS) (46.9%). Twenty-six patients were tested for a proto-oncogene B-Raf V600E (BRAF) mutation (18 had the mutation and 8 did not). The presence of a BRAF mutation was associated with 18F-FDG–avid CNS disease (P = 0.0357), higher SUVmax (P = 0.0044), and greater mortality (P = 0.0215). The presence of CNS disease had 88% specificity and a 92% positive predictive value for predicting the presence of a BRAF mutation. PET/CT examination results influenced patient management in 48% of cases (34/71). Conclusion: 18F-FDG PET/CT results may act as a biomarker for the presence of a BRAF mutation, aid in establishing a diagnosis, guide biopsies, and gauge the treatment response in ECD patients. Axial and pelvic skeletal involvement is greater than previously reported.
Erdheim–Chester disease (ECD) is a systemic histiocytosis that can involve several organs, with severity ranging from occult to life-threatening. The disease was first described by William Chester in 1930 after working with Austrian pathologist Jakob Erdheim (1). Even today, a correct diagnosis of ECD often takes years, given the rarity and variable manifestations of ECD.
Historically, there have been 3 broad categories of histiocytosis: malignant, Langerhans cell histiocytosis (LCH), and non–Langerhans cell histiocytosis. A modern 5-group scheme classifies ECD with LCH into the “L group” (2). Histologically, ECD has xanthomatous features, fibrosis, and occasional Touton giant cells. ECD histiocytes stain positive for CD68 and negative for CD1a, langerin, and S100 (rarely ECD can stain faintly positive for S100) (3).
Recently, the presence of a BRAF mutation in ECD patients was discovered (4). Some reports suggested that BRAF mutations are universally present in ECD, but most indicated about 50% involvement (2,5). BRAF mutations are associated with various malignancies, including pulmonary adenocarcinoma, melanoma, papillary thyroid and colorectal carcinomas (6–11). For this reason, ECD has been classified as a malignant disorder (12). The presence of a BRAF mutation narrows the diagnosis toward ECD and LCH, excluding all other types of histiocytosis (13). Treatment of ECD is also guided by BRAF status. Patients without a BRAF mutation (BRAF−) tend to have a moderately favorable response to cladribine (14), whereas patients with a BRAF mutation (BRAF+) respond well to targeted therapy with vemurafenib (15–21).
The diagnosis of ECD is on the rise, with at least 750 reported cases to date (22), but diagnosis is challenging given the rarity and varied presentation of ECD. Common symptoms include dyspnea, bone pain, ataxia, and visual disturbance (3). Although skeletal lesions are almost universally present on imaging, only 50% of patients have bone pain (23).
Imaging plays a key role in diagnosing ECD. The sine qua non finding in ECD has been lower-extremity diametaphyseal long-bone osteosclerosis (24–27). The osseous findings are best identified using 99mTc-methylene diphosphonate scintigraphy (bone scan) and are often difficult to detect radiographically (28). Several other sites commonly involved include the central nervous system (CNS), orbits, lungs, cardiovascular system, and retroperitoneum (29–37). Radiography, CT, and MRI have been used for focused evaluation. However, morphologic imaging may not accurately depict the severity or extent of disease or the response to therapy. In contrast, 18F-FDG PET/CT has been shown to have value for the systemic evaluation and quantification of the severity of ECD (38–41)—yet the data are scant.
Here, we review the largest set of single-center 18F-FDG PET/CT examinations to date, in patients with pathologically confirmed ECD. The aims of this study were to retrospectively analyze the utility of 18F-FDG PET/CT for the diagnosis, management, and treatment of patients with ECD and to correlate disease patterns and 18F-FDG activity with clinical data, including patient demographics, presenting symptoms, mortality, laboratory, histologic, and genetic data.
MATERIALS AND METHODS
Patient Selection
An institutional review board–approved retrospective review of a single-center institutional database was performed; the need to obtain informed consent was waived. A list of patients who underwent 18F-FDG PET/CT (from February 15, 2007, through February 21, 2017) was cross-referenced with the following terms: erdheim, chester, histiocytosis, fibrosis, and nonlangerhan. Subjects who met the following criteria were included: a clinical diagnosis of ECD, a tissue diagnosis (biopsy or surgical excision) consistent with ECD, an age of greater than 18 y, and no active coexisting malignancy.
Chart Review
The following information was recorded: age, race, sex, chief complaint, type of tissue for pathologic review, BRAF mutation status and test method, serum C-reactive protein level erythrocyte sedimentation rate (within 30 d of 18F-FDG PET/CT), and treatment history. The impact of 18F-FDG PET/CT on patient management was determined by a chart review 6 mo after 18F-FDG PET/CT, with attention to clinical, surgical, imaging, and pathology reports. Five categories of impact were recorded: contributing to the initial diagnosis of ECD, directing a subsequent biopsy, supporting the initiation or escalation of therapy, supporting the deescalation of or a holiday from therapy, and supporting continuation of the current clinical strategy.
Imaging Protocol
All 18F-FDG PET/CT examinations were performed with 3-dimensional detectors according to standard oncologic clinical protocols. All patients fasted for at least 6 h and had blood glucose levels of less than 200 mg/dL at the time of the examination. Images were acquired 60–70 min after the intravenous administration of 370–555 MBq (10–15 mCi) of 18F-FDG. Patients were imaged with the arms up if possible, covering at least the orbits to the midthighs (128 × 128 matrix, 3–5 min/bed position, depending on the body mass index). Low-dose CT was performed for attenuation correction and anatomic localization.
Imaging Review
MIM software (MIM Software Inc.) was used for image review. All examinations were reviewed in consensus by a nuclear radiology fellow and a board-certified nuclear radiologist (5 y of clinical experience). SUVs (SUVmax) were derived from body weight and volumes of interest, incorporating the most metabolically active disease. 18F-FDG–avid disease was considered present if uptake was visually greater than that of normal adjacent structures or the blood pool, when appropriate. Sites of 18F-FDG–avid disease were grouped into the CNS, orbital, paranasal sinus, pleural, cardiac/pericardiac, vascular, retroperitoneal, renal, skeletal, mesenteric/peritoneal, and gonadal; other, less common sites of disease were noted individually. For SUV analysis, including between-group comparisons (BRAF+ vs. BRAF−), only examinations obtained without treatment in the preceding 6 mo were included. Contributory CT findings were also recorded. A subset of 13 patients who underwent 18F-FDG PET/CT for both staging and restaging was evaluated using PET Response Criteria in Solid Tumors guidelines (42). The remaining studies could not be evaluated using these guidelines because of incomplete imaging data, treatment before initial examination, or delay between initial scan and therapy initiation.
Statistical Analysis
Data were analyzed using JMP software for Macintosh (JMP Pro, version 11.2.1; SAS Institute Inc.). Continuous variables were expressed as mean and SD. Categoric variables were expressed as absolute and relative frequencies. The P values for between-group comparisons of continuous data were calculated from a Kruskal–Wallis 1-way ANOVA. For categoric variables, P values were computed from contingency tables using the Fisher exact test. Statistical significance was established for P values of less than 0.05.
RESULTS
Patient Population and Demographics
Of the patients meeting the initial inclusion criteria, 2 were excluded because the clinical and pathologic presentation was mixed ECD and Rosai–Dorfman disease. Thirty-two patients (12 women and 20 men) were included; their mean age at the time of the first 18F-FDG PET/CT examination was 60.1 y (range, 32–83 y), 91% (29/32) were white, 6% (2/32) were Asian, and race was not reported for 3% (1/32). Common comorbidities included renal failure (25% [8/32]) and diabetes insipidus (31% [10/32]). Mortality secondary to ECD at the time of data collection was 12.5% (4/32). The most common presenting symptoms were dyspnea (28% [9/32]), bone pain (19% [6/32]), and visual disturbance (12.5% [4/32]).
Twenty-six subjects were tested for a BRAF mutation; 18 had the mutation and 8 did not. The BRAF mutations were substitutions of valine for glutamate at codon 600 (V600E) in all patients but 1, who had a V471F mutation. Methods of BRAF mutation analysis included immunohistochemical staining (9 positive and 6 negative), polymerase chain reaction (2 positive and 2 negative), next-generation genomic profiling (2 positive and 1 negative), and urinary cell-free DNA (8 positive and 2 negative). Some patients underwent more than 1 type of test to confirm BRAF status.
Imaging Results
Seventy-one 18F-FDG PET/CT examinations were performed for 32 patients over a 10-y period. The number of examinations showed an upward trend over time: 1 in 2007, 2 in 2008, 2 in 2009, none in 2010, 2 in 2011, 3 in 2012, 6 in 2013, 12 in 2014, 16 in 2015, 21 in 2016, and 6 in the first quarter of 2017. The average SUVmax of the single most 18F-FDG–avid lesion among all patients was 9.2 (SD, 6.1; range, 2.0–34.0).
The skeletal system was most commonly involved (91% [29/32]), with an average SUVmax of 4.5 (range, 1.5–15.7). Most cases involved the appendicular long bones, particularly around the knees. However, 47% of patients (15/32) had involvement of the axial skeleton and pelvis, including 6% of patients (2/32) with spinal involvement. Although appendicular long-bone disease was most frequently diametaphyseal, epiphyseal involvement was also seen. One case showed greater epiphyseal and patellar involvement (Fig. 1A). Disease involving small bones of the feet was identified in 9% of patients (3/32) (Fig. 1B).
(A) 18F-FDG PET/CT with coronal/axial fused and axial CT images of knees demonstrates predominantly patellar and epiphyseal 18F-FDG–avid disease with associated sclerosis. (B) Maximum-intensity-projection PET image in different patient illustrates diffuse 18F-FDG avidity involving small bones of feet.
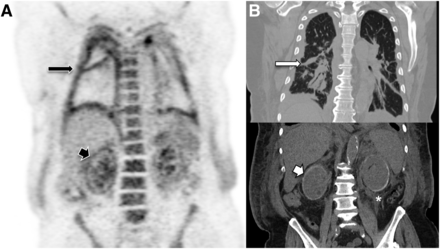
The kidneys were the second most common site of disease (81% [26/32]), with a mean SUVmax of 5.2 (range, 2.2–15.6). Renal disease was defined as increased 18F-FDG avidity within the renal cortex or capsule. The CT component of 18F-FDG PET/CT added confidence in identifying renal disease, with perinephric fat stranding (hairy kidney), poor corticomedullary differentiation (featureless kidney), and a rim of cortical calcification (“goose egg” kidney) (Fig. 2).
71-y-old man with shortness of breath. (A) Coronal PET demonstrates increased pleural (long black arrow) and renal (short black arrow) 18F-FDG activity. (B) Corresponding coronal CT depicts pleural thickening (long white arrow), bilateral perinephric fat stranding (hairy kidney) (*), and renal cortical calcification (goose egg kidney) (short white arrow).
The CNS was the third most common site of disease (47% [15/32]), with a mean SUVmax of 10.7 (range, 3.8–34). CNS disease occurred throughout the neural axis and involved the cerebrum, cerebellum, pons, and spinal cord. Disease involving the spinal cord (12.5% [4/32]) and lumbosacral nerves (6% [2/32]) was evident only on the PET component of the examination. A solitary case of 18F-FDG–avid suprasellar disease was identified. Extraaxial disease presented as thick plaquelike soft tissue, layered along dural membranes (Fig. 3). Suprasellar, filum terminale, and nerve root lesions were also identified.
50-y-old man with left mastoid pain. (A) Sagittal fused 18F-FDG PET/CT demonstrates 18F-FDG–avid disease within pons (thin white arrow) and along tentorium (thick white arrow). (B) Corresponding contrast-enhanced MRI illustrates infiltrative pons lesion (thin white arrow) and enhancing soft-tissue thickening along tentorium (thick white arrow).
Pulmonary parenchymal or pleural disease occurred in 41% of patients (13/32), with a mean SUVmax of 4.3 (range, 1.9–8.9). This finding predominantly involved the pleura and subpleural regions (Fig. 2). On CT, this presentation was most commonly manifested as smooth pleural and interlobular septal thickening.
Testicular hypermetabolism—some of which extended along the spermatic chord—was discovered in 40% of our male patients (8/20), with a mean SUVmax of 7.6 (range, 5.3–10.2). However, only 1 of these patients had associated scrotal symptoms.
Paranasal sinus disease was common (41% [13/32]), with an average SUVmax of 5.7 (range, 2.6–10). Most such disease occurred in the maxillary sinuses. The appearance of paranasal disease varied from mild mucosal 18F-FDG avidity and a normal CT appearance to marked mucosal thickening, hypermetabolism, and osteoneogenesis.
Orbital disease occurred in 38% of patients (12/32), with a mean SUVmax of 5.4 (range, 3.0–10.9). CT changes ranged from subtle focal soft-tissue thickening to bulky bilateral intraconal soft tissue enveloping the optic nerve (Fig. 4). There were 2 cases of globe disease—1 with bilateral diffuse scleral thickening and low-level 18F-FDG avidity and the other with marked bilateral choroidal thickening and intense hypermetabolism.
59-y-old man with orbital pain. (A) Axial fused 18F-FDG PET/CT demonstrates 18F-FDG–avid soft tissue encasing intraconal spaces (arrow). (B) Corresponding axial T1-weighted fat-saturated contrast-enhanced MRI depicts enhancing intraconal soft tissue (arrow).
Vascular disease—most commonly involving the aortic arch—occurred in 38% of patients (12/32), with an average SUVmax of 4.5 (range, 2.4–8.8). The heart and pericardium were involved in 34% of patients (11/32), with a mean SUVmax of 5.5 (range, 3.6–8.1).
Disease involving the retroperitoneal fascial planes, peritoneum, and central mesentery occurred in 34% of patients (11/32), with a mean SUVmax of 4.7 (range, 2.8–11.5). Lesions involving these sites appeared as plaquelike soft-tissue thickening. Mesenteric involvement had a perivascular predilection, commonly presenting as 18F-FDG–avid soft tissue encasing the central vascular pedicle.
Two cases of female breast involvement presented as diffuse bilateral soft-tissue thickening and hypermetabolism. There were 2 cases of hepatobiliary disease—1 involving the hepatic parenchyma and the other tracking along the portal triad. We also discovered ECD involving the space of Retzius and the pancreas.
Only 2 patients had 18F-FDG–avid lymph node disease, and these cases were isolated to regional nonenlarged lymph nodes with low-level hypermetabolism (SUVmax, 2.0–3.5). Neither was pathologically proven ECD. Both cases involved thoracic lymph nodes in the setting of active pulmonary ECD.
On the basis of PET Response Criteria in Solid Tumors methodology, 2 patients had a complete metabolic response—1 involving vemurafenib (BRAF+) and 1 involving anakinra (BRAF status unknown). Five patients had a partial metabolic response—3 involving vemurafenib (BRAF+), 1 involving pegylated interferon (peginterferon) (BRAF status unknown), and 1 involving prednisone (BRAF status unknown). Five patients had stable metabolic disease—1 involving vemurafenib (BRAF+), 2 involving peginterferon (BRAF−), and 2 involving cladribine (BRAF status unknown). One patient had progressive metabolic disease after peginterferon treatment (BRAF status unknown).
18F-FDG PET/CT examination results affected patient management in 48% of cases (34/71). Eight examinations (11%) contributed to the initial diagnosis of ECD. Eight examinations (11%) supported a watchful waiting approach. Seven examinations (10%) contributed to the escalation of therapy. Five examinations (7%) directed biopsy. Two examinations (3%) supported continuation of therapy. One examination (1.4%) led to deescalation of therapy, whereas another examination (1.4%) ruled out a suspected pulmonary malignancy.
Significant correlations between 18F-FDG PET/CT findings and BRAF+ status were identified. BRAF+ patients had higher mortality (P = 0.0215) and more frequent 18F-FDG–avid CNS disease (P = 0.0357) than BRAF− patients. 18F-FDG–avid CNS disease was able to predict the presence of a BRAF mutation with a sensitivity of 61%, a specificity of 88%, a positive predictive value of 92%, and a negative predictive value of 50%. No other correlations between BRAF+ status and pattern of disease were identified. However, patients with BRAF+ status had significantly higher SUVmax at their most metabolically active site of disease (9.7 [SD, 3.7]) than patients with BRAF− status (5.3 [SD, 2.2]) (P = 0.0044). The higher SUVmax of bone disease in BRAF+ patients also trended toward significance (4.5 [SD, 2.0] vs. 3.0 [SD, 1.3]; P = 0.0914).
There were no correlations between BRAF status and C-reactive protein level or erythrocyte sedimentation rate. However, patients with 18F-FDG–avid mesenteric/peritoneal disease had higher C-reactive protein levels (55.2 [SD, 11.0] mg/L) than those without it (18.7 [SD, 8.2] mg/L) (P = 0.0121).
DISCUSSION
To our knowledge, we have reviewed the largest number of 18F-FDG PET/CT examinations in a cohort of patients with histologically proven ECD. Correlations were discovered for BRAF+ status, intensity of 18F-FDG–avid disease, and presence of CNS disease. About 50% of the 18F-FDG PET/CT examinations affected patient management. A small number of examinations (10%) provided an initial benefit, either contributing to a diagnosis or guiding biopsy. Most examinations (40%) affected management at follow-up, supporting prior reports highlighting the value of 18F-FDG PET/CT in restaging (39). We observed increasing use of 18F-FDG PET/CT for patients with ECD, perhaps indicating that referring physicians perceived a greater value of 18F-FDG PET/CT.
Determining BRAF status in patients with ECD is an important branch of the clinical decision tree. Consensus guidelines recommend confirming negative BRAF results with more than 1 test modality or more than 1 sampled site (3). However, obtaining enough tissue for BRAF testing can be challenging, given tissue with a high ratio of fibrosis to histiocytes. Further, demineralization of bone samples impairs genetic analysis. Our data suggest that 18F-FDG–avid CNS disease and higher SUVmax may act as biomarkers for BRAF positivity. Higher SUVmax has also been associated with BRAF+ status in papillary thyroid carcinoma, suggesting a common mechanism between BRAF mutations and hypermetabolism (43).
18F-FDG PET/CT may be the most useful imaging modality for differentiating ECD from other histiocytoses. Given multiorgan involvement, ECD often elicits a broad differential diagnosis, including retroperitoneal fibrosis, IgG4-related disease, sarcoidosis, and other types of histiocytoses (44). A BRAF+ status essentially excludes other forms of non–Langerhans cell histiocytoses (45). Although lymph node involvement in ECD has been reported, it is rare (27,46,47). We confirmed this rarity, as only 2 patients (6%) in our cohort had mildly 18F-FDG–avid lymph nodes, which may have been reactive given concomitant pulmonary disease. Moderate to high lymph node 18F-FDG avidity (especially in more than 1 region) is therefore unlikely to be ECD related. In contrast, in Rosai–Dorfman disease, lymphadenopathy is a defining feature (48). ECD and LCH may involve the same organs. However, pulmonary disease and dermal disease are more characteristic of LCH, whereas cardiac involvement is a distinguishing feature of ECD (49). Along these lines, just over one-third (11/32) of our subjects had manifestations of 18F-FDG–avid cardiac or pericardiac disease.
The disease distribution of ECD can be wide and difficult to predict. Our data support previous reports of disease involving nearly every organ system, including hepatobiliary, bowel, and large arteries (50–52). With a disease so varied, “unusual” may be the norm for ECD. Our data support the previous recommendation of a vertex-to-toe protocol in 18F-FDG PET/CT scans for ECD patients (3).
Little information exists on 18F-FDG PET/CT of CNS disease in ECD. Our findings support a prior report of intra- and extraaxial CNS involvement, including the cerebrum, cerebellum, brain stem, spinal cord, dura, and suprasellar space (21). Although MRI is likely more sensitive for detection and exact localization, our findings support a prior report demonstrating 18F-FDG PET/CT utility in tracking the CNS ECD response to therapy (21).
In the present study, the most characteristic vascular presentation was 18F-FDG–avid soft tissue “coating” the aorta, corroborating a prior report of perivascular ECD involving the adventitial layer (Fig. 5) (53). We also found cardiac disease to have a right atrial propensity, similar to that previously reported (54).
(A) Axial fused 18F-FDG PET/CT of thorax demonstrates intensely 18F-FDG–avid soft-tissue thickening surrounding aortic arch (arrow). (B) Corresponding contrast-enhanced CT demonstrates asymmetric soft tissue involving aortic arch in expected region of adventitia (arrow).
Axial and pelvic skeletal involvement of ECD was once deemed rare (55). However, 47% of patients in our cohort (15/32) had axial or pelvic skeletal involvement, albeit generally less severe than appendicular disease (Fig. 6). The higher rate of axial disease in the present study may have been partly secondary to improvements in 18F-FDG PET/CT technology. We also identified unique patterns of osseous ECD, with 9% of patients (3/32) showing involvement of the small bones of the feet and 1 patient showing predominantly epiphyseal/patellar disease. A prior report of an adolescent with ECD suggested that 18F-FDG avidity may occur before radiographic evidence of sclerosis (56). Some cases from our cohort support this notion. However, we were unable to find a case of 18F-FDG–avid osseous disease without a corresponding abnormal bone scan (Fig. 7).
63-y-old woman with back pain. (A) Fused sagittal PET/CT image of spine demonstrates multifocal 18F-FDG–avid skeletal disease. (B) Contrast-enhanced fat-saturated T1-weighted sagittal MRI illustrates numerous enhancing lesions throughout spine.
(A) Coronal 18F-FDG PET/CT of distal lower extremities demonstrates 18F-FDG–avid disease within both tibias and distal femur (arrows) without osteosclerosis. (B) Anterior planar 99mTc-methylene diphosphonate whole-body bone scan demonstrates increased uptake throughout both distal lower extremities (bracket).
The limitations of the present study included its retrospective nature and small sample size. Multiple methods were used to determine BRAF status; these methods had inherently different detection accuracies. Although many patients had received prior therapy, when we correlated 18F-FDG PET/CT examinations with clinical data, we attempted to control for treatment effect by including only examinations from patients who had not received active treatment in the preceding 6 mo.
CONCLUSION
18F-FDG PET/CT results may act as a biomarker for the presence of a BRAF mutation, aid in establishing a diagnosis, guide biopsies, and gauge the treatment response in ECD patients. Axial and pelvic skeletal involvement is greater than previously reported.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
Footnotes
Published online Nov. 2, 2017.
- © 2018 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication August 15, 2017.
- Accepted for publication October 12, 2017.