Abstract
In nuclear medicine, the term theranostics describes the combination of therapy and diagnostic imaging. In practice, this concept dates back more than 50 years; however, among the most successful examples of theranostics are peptide receptor scintigraphy and peptide receptor radionuclide therapy of neuroendocrine tumors. The development of these modalities through the radiolabeling of somatostatin analogs with various radionuclides has led to a revolution in patient management and established a foundation for expansion of the theranostic principle into other oncology indications. This article provides a review of the evolution and development of the theranostic radionuclide approach to the management of neuroendocrine tumors, as described by the inventor of this technique, Eric P. Krenning, in an interview with Rachel Levine.
Although the term theranostics was reported to be coined by John Funkhouser in 1998 (1) to describe a material that combines the modalities of therapy and diagnostic imaging, this basic principle had been applied to imaging and treating thyroid diseases for more than 50 years (2). In 1941, Saul Hertz was the first to use 131I therapeutically in patients with hyperthyroidism and later those with thyroid cancer (2). In 1951, the U.S. Food and Drug Administration (FDA) approved sodium iodide (131I) for use in patients with thyroid disease. It was the first FDA-approved radiopharmaceutical (3).
PEPTIDE RECEPTORS AND NEW THERANOSTIC APPLICATIONS
Among the most successful examples of the theranostic concept in nuclear medicine are peptide receptor scintigraphy (PRS) and peptide receptor radionuclide therapy (PRRT) for imaging and treating cancer. These innovations were first used in patients with mainly neuroendocrine tumors (NETs) (4,5) in the late 1980s and early 1990s, although these acronyms were not published until 1994 (5).
The origination of PRS and PRRT date back to an endocrinology postdoctoral meeting of Erasmus University Medical Center (Erasmus MC), Rotterdam, The Netherlands, in 1985. Steven Lamberts presented slides with receptor autoradiograms obtained and originating from Jean Claude Reubi, who was, at that time, working at the Sandoz Research Institute in Basel, Switzerland. Using slices of tumor tissue obtained from patients with gastroenteropancreatic NETs (GEP-NETs), Reubi, Lamberts, and collaborator Larry Kvols (at the Mayo Clinic, Rochester, Minnesota, at that time) had demonstrated, for the first time, the presence of receptors for somatostatin on the surface of intestinal NET cells. This finding was crucial to identifying (one of) the mechanisms of action of octreotide, a somatostatin analog invented at the Sandoz Research Institute and first published in 1982 (6,7). Reubi’s team was using this analog coupled to the iodine isotope 125I (8). Eric P. Krenning, an endocrinologist and then recently appointed head of nuclear medicine at Erasmus MC, was present at that meeting. Working daily with the isotopes 123I and 131I for the localization and treatment of thyroid cancer, Krenning instantly recognized the potential of radiolabeled peptides to localize and treat NETs in a clinical setting (“from bench to bedside”). Later that evening, at the usual social gathering after the scientific part of the meeting, Krenning started a discussion with chemists Theo Visser and Roel Docter regarding how to change the application of the somatostatin analog from in vitro receptor autoradiography to in vivo receptor scintigraphy by radiolabeling it with the 2 iodine isotopes frequently used in nuclear medicine, namely, 123I and 131I.
CHALLENGE OF RADIOLABELING
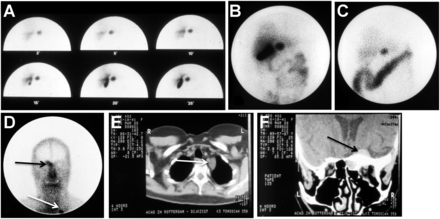
After a period of trial and error (resulting in, “Eric, it is a no-go”) during attempts to label the peptide with a radioiodine isotope appropriate for imaging in humans, Willem Bakker and Wout Breeman, chemists working in Krenning’s department, made a final and crucial attempt. They ordered every available preparation of 123I from vendors worldwide and finally found a single, high-specific-activity preparation of 123I that enabled them to overcome the labeling challenge and succeed in producing a suitable radiolabeled somatostatin analog, 123I-labeled Tyr3-octreotide. In 1987, the surprising and exciting planar and SPECT images obtained of a GEP-NET patient with this new technique showed almost instantly the primary gastrinoma, an unknown metastasis in the Virchow node, and a known meningioma (Fig. 1). By 1990, [123I-Tyr3]octreotide had been used in several hundreds of patients to localize carcinoid tumors, pancreatic endocrine tumors, and paragangliomas (9). Unfortunately, concentrated intestinal accumulation resulting from the high biliary excretion of 123I hampered the interpretation of planar and SPECT images of lesions in the abdomen. This drawback was serious and provided evidence that a better agent was needed. Moreover, the high cost and limited availability of the highly specific form of 123I required to radiolabel the analog made it impractical.
[123I-Tyr3]octreotide planar scintigraphy of gastrinoma in 1987. (A–D) Abdominal images show gallbladder and primary tumor at 2–25 min after injection (A) and at 6 h (B) and 24 h (C) after injection, with prominent bowel accumulation, as well as unknown metastasis in Virchow node (white arrow) and known meningioma (black arrow) (D). (E and F) CT images show Virchow node (E) and meningioma (F).
NEW SOLUTION EMERGES: 111IN-PENTETREOTIDE
By 1990, Krenning’s team, in cooperation with colleagues at Sandoz Research Institute (Janos Pless, Rainer Albert, Christian Bruns, Peter Marbach, and Barbara Stolz), had successfully developed 111In-pentetreotide (OctreoScan; Mallinckrodt). In 1993, Krenning’s team published data on their experiences using 111In-pentetreotide imaging in more than 1,000 patients (10). This publication is now widely considered an important reference in nuclear medicine and is the most frequently cited paper from the European Journal of Nuclear Medicine. In 1994, the FDA approved 111In-pentetreotide as an imaging radiopharmaceutical, exclusively on the basis of results achieved in approximately 350 European patients because its sensitivity and specificity in patients with GEP-NETs were higher than those of CT or MRI. 111In-pentetreotide was the first peptide-based radiopharmaceutical ever approved.
Having established a simpler and more effective imaging technology for NETs (especially for abdominal imaging), the next logical step for Krenning and his team was to follow the model of radioiodine use in thyroid cancer and hyperthyroidism to create a theranostic agent. The team worked closely with Mallinckrodt Medical, Petten, The Netherlands, the company that had licensed and was actively marketing OctreoScan, to create PRRT using very high doses of the product. In 1992, using the specific physical characteristics of the Auger and conversion electrons of 111In, the Erasmus MC team successfully treated the first NET patient (with glucagonoma) with high doses of 111In-pentetreotide (5).
NEXT STEPS FOR PRRT
After a few years of PRRT experience with 111In-pentetreotide (Fig. 2) (11), it became clear that other radionuclides might be better suited for the regimen than 111In because its short tissue range resulted in relatively modest tumor shrinkage (based on anatomic imaging with CT/MRI). In addition, DOTA-chelated peptides, which could be more easily labeled with radioactive metals, also started becoming available (12–14).
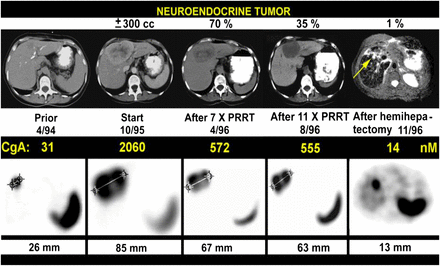
PRRT with very high doses (7.4 GBq [200 mCi] per cycle) of 111In-pentetreotide in patients with NETs in 1994–1996. Tumor shrinkage and tumor marker decline (as indicated by chromogranin A [CgA]) after 11 cycles were 65%–70% (top row); subsequent hemihepatectomy left behind small tumor of 9 mm at cutting edge. Top row shows CT (except for 11/96 [MRI]), and bottom row shows transverse slices from 111In-pentetreotide SPECT. (Adapted with permission of (11).)
In retrospect, Krenning observed, minimal shrinkage of tumors (based on CT/MRI) with the achievement of stable disease may not necessarily have been a less-than-optimal result because stable disease may indicate that fibrosis has replaced cancer—a distinction not made by CT/MRI. Such a distinction can be made only with functional imaging, such as PET. This observation is further supported by similar progression-free survival and overall survival data obtained from a comparison of GEP-NET patients with stable disease as a treatment outcome to patients with partial or complete remission as a treatment outcome (15).
Despite its seemingly limited impact on tumor shrinkage (based on CT/MRI), PRRT with 111In-pentetreotide did have an impressive positive effect on the quality of life of patients, including those with large tumors. Krenning observed that 2 wheelchair-bound patients were able to walk again after this therapy and recalled a similarly impressive finding shared (written communication, March 18, 2007) by Richard Baum’s group at Zentralklinik, Bad Berka, Germany, after 90Y PRRT treatment of a young boy with malignant paraganglioma. The overall improvement in the quality of life of patients after PRRT treatment was so significant that patients were demanding repeated treatments with 111In-pentetreotide (in the absence of an alternative treatment modality at the time), despite the acknowledged risk of developing myelodysplastic syndrome or leukemia. Indeed, a total of 50 patients received many cycles of 111In-pentetreotide in about 5 y at Erasmus MC. Three of the 6 patients who received more than 100 GBq (2,700 mCi) (equivalent to 450 adult imaging doses) cumulatively developed myelodysplastic syndrome or leukemia at a mean bone marrow dose of about 3 Gy (11).
DEVELOPMENT OF KIDNEY PROTECTION WITH LYSINE AND ARGININE
With the introduction of 90Y PRRT, it became apparent that measures to protect against renal uptake of the radionuclide were needed. After an initial publication on the protective properties of certain amino acids by Hammond et al. (16), Krenning, Marion de Jong, Edgar Rolleman, and Roelf Valkema started preclinical and clinical investigations to optimize kidney protection with the amino acids lysine and arginine during PRRT with somatostatin analogs. After significant testing in patients receiving 111In PRRT, optimal and safe doses (not inducing hyperkalemia) of the amino acids were achieved. The discovery of the role of megalin (a multiligand scavenger receptor in renal proximal tubules) in the mechanism of action of lysine facilitated this success (17). The Erasmus MC lysine–arginine formulation, which is still used worldwide today, contains 25 g of each of these amino acids in only a 1-L volume. This low volume provides an advantage over the larger volumes of commercially available amino acid solutions with similar amounts of lysine and arginine being given to NET patients during a 4-h infusion. These patients often have heart valve abnormalities and thus are more prone to heart failure after receiving such a large fluid load in a short time. This specialized formulation is also an improvement over commercially available amino acid solutions because the latter tend to induce significant nausea and vomiting in patients more frequently.
[90Y-DOTA,TYR3]OCTREOTIDE ENTERS SCENE
In 1997, Novartis, in Basel, Switzerland, launched a study of a new type of PRRT—PRRT with 90Y-labeled DOTA,Tyr3-octreotide (DOTATOC, edotreotide, or OctreoTher [Novartis])—in collaboration with Cliniques Universitaires St. Luc, Brussels, Belgium (Stanislas Pauwels, Francois Jamar, and Stefan Walrand); the University of New Mexico, Albuquerque, New Mexico (Larry Kvols); and Erasmus MC (Eric P. Krenning and Roelf Valkema). 90Y was considered an alternative to 111In because of its longer range of tissue penetration, which was hoped to have a greater impact on tumor shrinkage. The protocol for the study, named B151, was unique in that patients were required to undergo PET dosimetry through another trial protocol (B101) before receiving PRRT. The Brussels group successfully introduced 86Y-labeled DOTATOC PET dosimetry in the B101 study (18–22), whereas the other European centers used 111In-labeled DOTATOC as a 90Y surrogate for dosimetry. The isotopes 86Y and 90Y are positron- and β-emitters, respectively.
The objectives of the 86Y-labeled DOTATOC PET study were to assess the pharmacokinetics and biodistribution of this radiopeptide, to estimate the individual maximum tolerated doses of 90Y-labeled DOTATOC (by applying specific kidney and bone marrow radiation limits), to evaluate the effects of commercially available amino acid solutions on kidney protection, to measure the effect of the peptide mass on tumor accumulation, and to compare 90Y-labeled DOTATOC dosimetry with that of 111In-pentetreotide (18–22). The overall response rates obtained with 90Y-labeled DOTATOC PRRT at most of the European sites were indeed better than those obtained with 111In-pentetreotide; however, this increase in response rates came at the expense of higher renal toxicity.
FIRST GALLIUM-LABELED PEPTIDE IMAGING
In 1995 and early 1996, the Basel group, in cooperation with Heiner Bihl (Katharinenhospital, Stuttgart, Germany), imaged 12 patients with 67Ga-DOTATOC γ-camera imaging as a surrogate for 68Ga-DOTATOC PET. The clinical data were presented at the European Association of Nuclear Medicine annual meeting in 1996 and were included in the highlight lecture (23).
90Y PRRT CONTINUES TO THRIVE
In the late 1990s and early 2000s, “early adopters” of PRRT were successfully using 90Y coupled to various somatostatin analogs (13,24–27). A clinical study with 90Y-DOTATOC was started in Basel in June 1996; soon thereafter, the study became a collaboration with the European Institute of Oncology, Milan, Italy (Giovanni Paganelli and Lisa Bodei), and the University Hospital Frankfurt, Frankfurt am Main, Germany (Richard Baum). In 1998, Otte et al. (28) reported the use of 90Y-labeled DOTATOC in the treatment of 10 patients with different somatostatin receptor–positive tumors. In 2001, the results of a phase 2 study of 90Y-DOTATOC in 41 patients with GEP-NETs and bronchial tumors demonstrated an overall response rate of 24% and a significant reduction in carcinoid syndrome in 83% of the patients (26). In 2011, the results of a study by Imhof et al. of 1,109 patients showed a morphologic response in 34.1% of the patients, longer survival in 79% of the patients, and grade 4 or 5 permanent renal toxicity in 9.2% of the patients (29).
The primary observed disadvantage of 90Y PRRT was renal toxicity. 90Y PRRT resulted in renal failure in patients when performed without kidney protection, although some renal toxicity was still observed even with the administration of amino acids for kidney protection (30–32). These findings led to the conclusion that renal protection is imperative for any PRRT with β-emitting radionuclides. Therefore, even the first patients receiving [177Lu-DOTA,Tyr3]octreotate PRRT in 2000 were given an amino acid solution for renal protection.
177LU PRRT IS DEVELOPED
In 1998 (33), a unique collaboration named Specific Peptides for Imaging and Radio Isotope Therapy (S.P.I.R.I.T.) was established by several intercontinental universities: Cliniques Universitaires St. Luc, Brussels, Belgium (Stanislas Pauwels and Francois Jamar); University of Berne, Berne, Switzerland (Jean Claude Reubi); University Hospital Basel, Basel, Switzerland (Helmut Maecke); Erasmus MC, Rotterdam, The Netherlands (Eric Krenning, Marion de Jong, and Theo Visser); University of New Mexico, Albuquerque, New Mexico (Larry Kvols); and Mallinckrodt Medical/Covidien, Petten, The Netherlands, and St. Louis, Missouri (Jack Erion, Ananth Srinivasan, Joe Bugaj, Michelle Schmidt, Jean-Luc Vanderheyden, and Geert Ensing). The goal was to develop marketable radiopharmaceuticals using targeting peptides and peptidelike molecules to deliver diagnostic or therapeutic medical doses to specific sites within the body.
One of the peptides originating from this network was [177Lu-DOTA,Tyr3]octreotate (LuTate or 177Lu-DOTATATE), an investigational therapy invented by the Mallinckrodt Medical researchers (14). The first clinical studies with [177Lu-DOTA,Tyr3]octreotate started in 2000 in Rotterdam, The Netherlands, and involved a prospectively designed clinical protocol that later formed the basis of a multinational phase 3 trial named NETTER-1 (34).
In 2003, a study of [177Lu-DOTA,Tyr3]octreotate therapy in 35 patients with GEP-NETs demonstrated complete remission in 1 patient (3%), partial remission in 12 patients (35%), stable disease in 14 patients (41%), and progressive disease in 7 patients (21%), including 3 patients who died during the treatment period (35). In 2005 and 2008, Kwekkeboom et al. published data illustrating tumor remission in high percentages of 131 and 310 patients, respectively, with metastasized or inoperable GEP-NETs treated with [177Lu-DOTA,Tyr3]octreotate (36,37), including improvements in various aspects of quality of life (38,39).
Clinicians at Erasmus MC were soon treating patients from countries all over the world, including patients from Europe, the United States, Brazil, Pakistan, and Australia. By August 2006, the Erasmus MC team (Dik Kwekkeboom, Jaap Teunissen, Boen Kam, and many others) had performed more than 1,750 treatments with [177Lu-DOTA,Tyr3]octreotate in more than 500 patients—and not only those with classic NETs (40,41). Over the years, that number has increased to more than 1,500 patients, even though [177Lu-DOTA,Tyr3]octreotate has not yet been approved by regulatory authorities. Such enormous referral from foreign countries also took place in Basel, Bad Berka, and Milan; treatment with [177Lu-DOTA,Tyr3]octreotate in the United States started in 2013 (42).
After more than 15 y of experience with up to 29.6 GBq (800 mCi) of cumulative administered doses of [177Lu-DOTA,Tyr3]octreotate, Erasmus MC clinicians concluded that routine individual dosimetry did not enhance the safety of a single patient, as long as a strict clinical protocol with careful monitoring and control of bone marrow function was followed during PRRT. In addition, overall, there was no observed dose–response effect on serious (permanent) bone marrow toxicity, and it was believed to be unlikely that the widely used bone marrow limit of 2 Gy had any validity with regard to this radioligand (43). Finally, this experience also demonstrated that PRRT with [177Lu-DOTA,Tyr3]octreotate, administered in conjunction with appropriate amounts of amino acids for kidney protection, had virtually no renal toxicity (44). In retrospect, Krenning noted, the cumulative dose initially selected in 2000 appeared to be correct—not surprisingly, however—considering that it was based on the global experience of more than 50 years of safe application of similar cumulative doses of 131I for the therapy of thyroid cancer and that 177Lu and 131I share many physical characteristics (30).
In 2001, BioSynthema Inc. was founded in St. Louis, Missouri (Jack Erion, Henk van Rossem, Mary Palank and Eric Krenning), as a spinoff of the S.P.I.R.I.T. collaboration to discover and develop pharmaceuticals targeting cell surface receptors overexpressed in cancer cells. In 2007, Covidien and BioSynthema Inc. signed exclusive agreements to develop cancer therapy with [177Lu-DOTA,Tyr3]octreotate (45). In 2010, BioSynthema Inc. was acquired by Advanced Accelerator Applications, S.A., Saint-Genis-Pouilly, France, a radiopharmaceutical company. With the support of Advanced Accelerator Applications, good manufacturing process–compliant manufacturing of [177Lu-DOTA,Tyr3]octreotate was established, a regulatory pathway was negotiated with the FDA and the European Medicines Agency, and a pivotal multinational phase 3 study (NETTER-1) was conducted at 41 global sites.
By 2015, the NETTER-1 study had met its primary endpoint of assessing progression-free survival, demonstrating that [177Lu-DOTA,Tyr3]octreotate significantly improved progression-free survival compared with octreotide acetate injection (Sandostatin LAR; Novartis; 60 mg) in patients with advanced midgut NETs (34). In January 2017, the results of this phase 3 trial of [177Lu-DOTA,Tyr3]octreotate in patients with midgut NETs were published in the New England Journal of Medicine (34). A new drug application and a marketing authorization application for [177Lu-DOTA0,Tyr3]octreotate are currently under review with the FDA and the European Medicines Agency, and more than 1,600 NET patients have received treatment with [177Lu-DOTA0,Tyr3]octreotate under compassionate use and named patient programs sponsored by Advanced Accelerator Applications in 11 countries.
Krenning holds the opinion that the results of the NETTER-1 trial validate previously published phase 2 trial data, making prospective phase 2 trials more impactful than generally thought in oncology. His interpretation is that the theranostic approach (“PRS positivity means PRRT feasibility”), as discussed here, is completely different from the general practice of chemotherapy. His hope is that future prospective phase 2 PRRT trials for other malignancies that express high levels of targetable peptide receptors, such as (castration-resistant) prostate cancer and breast cancer, may be sufficient for regulatory approval (46,47). To this end, approximately 15 different peptides (4,10,48–54) were investigated in humans at Erasmus MC between 1987 and 2009, and various different peptides and developments exploring the potential utility of PRRT in oncology and other diseases have been reported since then (see the following text).
FUTURE OF THERANOSTICS
The use of 90Y and 177Lu, as single agents or in combination, for PRRT continues in several centers throughout Europe because animal studies have suggested that larger tumors respond better to 90Y PRRT and smaller ones respond better to 177Lu PRRT. Unfortunately, the animal tumor model used is not representative of the nature of tumors in humans (heterogeneous vs. homogeneous distributions of somatostatin receptors). Additionally, there are currently no randomized trial data comparing the combination of 90Y PRRT and 177Lu PRRT with 177Lu PRRT alone, leaving the actual benefit of this combination of radionuclides in humans with GEP-NETs unknown, although potential efficacy has been reported (55,56).
Newer trends include combinations of [177Lu-DOTA0,Tyr3]octreotate therapy with chemotherapy, targeted agents, or immunotherapies. In 2007, the first randomized phase 3 trial comparing the combination of 177Lu PRRT and capecitabine (Xeloda; Genentech), an oral chemotherapy agent, with 177Lu PRRT alone was started at Erasmus MC, with data expected in 2017 (57). Much experience and success with this approach have been obtained in Australia, where combination treatment with PRRT and chemotherapy is called peptide receptor chemoradionuclide therapy (58–60).
PRS and PRRT have gained popularity among NET specialists worldwide as theranostic approaches for their patients, although until the Society of Nuclear Medicine and Molecular Imaging and the European Neuroendocrine Tumor Society published guidelines for their use, there were few standardized clinical practices for the administration and implementation of these procedures or the evaluation of patient progress (61,62).
In parallel with the advances in PRRT, investigators have been working on new ways to improve 111In-pentetreotide, the long-standing standard of care in PRS by SPECT (63). These new developments are primarily supported by the introduction of hybrid PET/CT cameras and 68Ga generators. The recent approvals by the FDA and the European Medicines Agency of [68Ga-DOTA,Tyr3]octreotate and edotreotide for the localization of NETs by PET/CT (64) have already significantly changed the field of NET imaging—more than 20 years after the approval of 111In-pentetreotide—and paved the way for additional theranostic pairings using 68Ga imaging and 90Y or 177Lu PRRT.
For example, the implementation of radiolabeled compounds targeting prostate-specific membrane antigen (PSMA) for both diagnostic and therapeutic applications is considered to be a milestone in the management of patients with castration-resistant prostate cancer (46,47). The observation of frequent, persistent PSMA expression in such patients has provided the rationale for the recent introduction of PSMA radioligand therapy, with very promising initial results (46,65).
Other recent developments include somatostatin antagonists with surprisingly higher levels of binding to NET lesions than classic agonists (66), pansomatostatin radioligands for multiple sst1–sst5 tumor targeting (67,68), and the use of specific enzyme inhibitors as radiopeptide escorts promoting tumor targeting (69).
One final innovation in the theranostic paradigm is the development of the NETest, a gene transcript measure, which is of interest as a possible predictor of PRRT outcome (70). It is hoped that such a test also may act as a prognostic factor for the induction of myelodysplastic syndrome or leukemia by PRRT (occurring in ∼2% of patients) because these disorders do not seem to be directly related to the bone marrow impact of current clinical PRRT regimens.
CONCLUSION
In looking back over the past 30 years of theranostic development and progress, Krenning emphasized the importance of teamwork, collaboration between academia and industry, and the lesson of perseverance. He hopes that the success of the NETTER-1 study and the potential approval of [177Lu-DOTA0,Tyr3]octreotate by regulatory authorities will translate into a breakthrough of support for the further investigation of peptides for use in diagnostics and PRRT for a wide range of cancer types. He also hopes that the long time and enormous amount of energy he spent exploring PRRT for NETs are not representative of the effort that will be required to develop PRRT for other cancers. According to Krenning, “Many lessons have been learned from the development of PRS and PRRT, which can be applied going forward. The success of NETTER-1 and enormous support from industry over the past 5 years has made it possible to gain worldwide attention for PRS and PRRT in various medical disciplines.”
DISCLOSURE
Both authors are shareholders of Advanced Accelerator Applications, and Rachel Levine is an employee of Advanced Accelerator Applications. No other potential conflict of interest relevant to this article was reported.
- © 2017 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication February 6, 2017.
- Accepted for publication April 13, 2017.