Abstract
The methylguanidine derivative 11C-GMOM (11C-labeled N-(2-chloro-3-thiomethylphenyl)-N′-(3-methoxyphenyl)-N′-methylguanidine) has been used successfully to quantify N-methyl-d-aspartate (NMDA) receptor binding in humans. The purpose of the present study was to estimate the 11C-GMOM radiation dose in healthy humans. Methods: After 11C-GMOM injection, 3 female and 2 male subjects underwent 10 consecutive whole-body PET scans in approximately 77 min. Seven source organs were defined manually, scaled to a sex-specific reference, and residence times were calculated for input into OLINDA/EXM software. Accepted tissue-weighting factors were used to calculate the effective dose. Results: The mean absorbed radiation doses in source organs ranged from 7.7 μGy·MBq−1 in the brain to 12.7 μGy·MBq−1 in the spleen. The effective dose (±SD) was 4.5 ± 0.5 μSv·MBq−1. Conclusion: The effective dose of 11C-GMOM is at the lower end of the range seen for other 11C-labeled ligands, allowing for serial PET scanning in a single subject.
The phencyclidine binding site within the pore of the glutamatergic N-methyl-d-aspartate (NMDA) receptor ion channel is a target for NMDA antagonists such as MK-801 and ketamine. Imaging the phencyclidine site using PET with radiolabeled antagonists has been pursued avidly, but clinical implementation of these radiotracers has been held back by high nonspecific binding, high lipophilicity, low brain entrance, or rapid radioligand metabolism (1). Results from human molecular imaging studies with methylguanidine derivatives such as 11C-CNS 5161 (2) and 18F-GE-179 (3) seem more promising. 11C-GMOM (11C-labeled N-(2-chloro-3-thiomethylphenyl)-N′-(3-methoxyphenyl)- N′-methylguanidine) studies in awake rats showed that administration of the antagonist MK-801 decreased tracer binding in brain regions of interest (ROIs), whereas the channel activator D-serine increased binding (4). Recent experiments in healthy subjects showed that intravenous administration of ketamine (0.3 mg·kg−1) reduced the 11C-GMOM inhibition constant (Ki) in total brain gray matter by, on average, 66% (5). More human studies with 11C-GMOM are planned, but at present no data on tracer distribution and radiation dose are available. Although various methods can be used to scale dose estimates from preclinical species to humans, potentially significant interspecies differences mean that extrapolation from rodent data should be considered with care (6,7). The purpose of the present study was to calculate the 11C-GMOM effective dose in men and women for use in future clinical PET protocols.
MATERIALS AND METHODS
Subjects and Scan Protocol
The study was approved by the Medical Ethics Review Committee of the VU University Medical Center Amsterdam, and all subjects signed an informed consent form before inclusion. Five healthy subjects were included, 2 men and 3 women, with a mean weight ± SD of 75.4 ± 7.0 kg, height of 177 ± 10 cm, and age of 24.9 ± 2.5 y. Subjects were screened, and health status was confirmed by blood and urine tests (complete blood count, serum chemistry, drug screen), together with a physical examination and medical history. The scanning protocol was identical to that reported previously (8). Subjects were positioned on the bed of a Gemini TF-64 PET/CT scanner (Philips), and a 35-mAs low-dose whole-body CT scan was acquired. 11C-GMOM was synthesized according to methods described previously (5). After intravenous injection of 376 ± 19 MBq of 11C-GMOM, a series of 10 whole-body sweeps was performed, taking 40 s per bed position and typically requiring 11 bed positions to cover the body from the top of the head to the upper thigh. Overlap between bed positions was approximately 50% to maintain a constant axial coverage. Total acquisition time was approximately 77 min, that is, 3.8 times the half-life of 11C. Five 0.5-mL venous blood samples per subject were taken manually at 10, 27, 44, 60, and 77 min after 11C-GMOM injection for measurement of whole-blood radioactivity concentrations.
Data Analysis
All PET scans were reconstructed using the standard time-of-flight reconstruction algorithm, including normalization, and corrections for scatter, randoms, attenuation, and dead-time (9). Non–decay-corrected radioactivity (Bq) in source organs was used to calculate 11C-GMOM residence times. Source organs (ROIs) were defined manually on relevant slices of either CT or PET images depending on optimal visibility of those organs. ROIs included heart, liver, kidneys, spleen, lungs, thyroid, and brain. Organ volume (mL) was derived automatically from the ROIs. The lung ROI was edited manually when its location on the respiration-averaged PET scan differed from that on the CT scan. Individual ROIs were projected onto each serially acquired PET frame and manually adjusted in the case of patient motion, and 11C-GMOM time–activity curves were generated. Radioactivity per organ volume (Bq·mL−1) was calculated assuming that the distribution of radioactivity within an organ was uniform. Time–activity curves were extrapolated from the last whole-body scan to infinity, assuming only physical decay and no further organ clearance. Red marrow activity concentration was assumed to be one third of the whole-blood radioactivity concentration (10). SUVs were calculated by dividing non–decay-corrected tissue radioactivity concentration by injected dose per body weight. 11C-GMOM residence times (i.e., normalized cumulated activities) in the 7 source organs were obtained through multiplication of the areas under the time–activity curves with each subject’s organ mass. Mass was calculated by scaling reference organ weights from a standard man (73.7 kg) or woman (56.9 kg) to each subject’s body weight using the software package OLINDA/EXM 1.1 (11). Residence times of the manually drawn source organs of each subject were entered into the software to calculate absorbed dose (μGy·MBq−1) for the target organs, 24 in total. Multiplication of absorbed doses with tissue-weighting factors gave the organ effective doses (μSv·MBq−1). The factors, which represent each organ’s relative risk contribution should the whole body be irradiated uniformly, were taken from the International Commission on Radiological Protection publication 103 (12). Total effective dose is the sum of the organ effective doses.
RESULTS
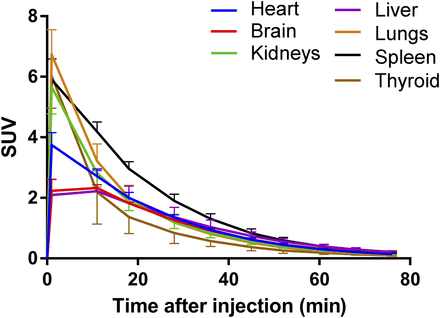
Figure 1 shows coronal slices of the 11C-GMOM distribution in a female subject as a function of time. Figure 2 shows subject-averaged SUVs for the manually delineated ROIs. In early time frames, 11C-GMOM concentrations are highest in the lungs, spleen, kidneys, and thyroid. Mean residence times are shown in Table 1. The longest residence time (0.0368 ± 0.0093 h) was observed in the liver, the shortest in the thyroid (0.0005 ± 0.0002 h). In all subjects, the organ with the highest absorbed dose was the spleen (mean, 12.7 μGy·MBq−1). The mean effective dose was 4.5 ± 0.5 μSv·MBq−1 (men, 4.3 ± 0.8 μSv·MBq−1; women, 4.6 ± 0.4 μSv·MBq−1).
Coronal PET/CT fusion images of 11C-GMOM uptake in Bq per mL tissue (BQML) showing tracer biodistribution at 4 different time points (2, 18, 36, and 52 min) for a female subject. Each panel is a composite of 11 bed positions of 40 s each.
Time–SUV curves (non–decay-corrected) showing 11C-GMOM SUV (mean and SD of 5 subjects) in manually delineated source organs.
11C-GMOM Residence Times (in Source Organs), Absorbed Organ Doses, and Effective Organ Doses
DISCUSSION
Organ radiation exposure for the NMDA receptor radiotracer 11C-GMOM was measured in 5 healthy subjects. The mean effective dose was 4.5 ± 0.5 μSv·MBq−1. Therefore, a PET scan after an injection of 370 MBq of 11C-GMOM would lead, on average, to a radiation dose of 1.67 mSv, which is in the range of other 11C-labeled tracers (7,13). An injected activity of 2,222 MBq would lead to a radiation dose of 10 mSv, which is the limit for proof-of-concept studies in healthy subjects according to International Commission on Radiological Protection 62 guidelines and The Dutch Commission on Radiation Dosimetry (14). For institutions following organ dose limits for radiopharmaceuticals that are administered under U.S. Radioactive Drug Research Committee regulations, the maximum injected activity per study would be limited by the critical organ rather than the (whole body) effective dose. The critical organ in this study was the spleen, with an absorbed dose of 12.7 μGy·MBq−1, which is equivalent to 4.7 mSv for a typical injection of 370 MBq of 11C-GMOM.
Radiotracer dose deposition in tissue depends on both the biologic half-life of the compound (and any radiolabeled metabolites) and the half-life of the radionuclide. Given the relatively short half-life of 11C (20.4 min), the effective dose mainly depends on organ perfusion and retention. Indeed, in the present study, the highest absorbed doses were found for highly perfused organs. Often, the urinary bladder wall is reported as being the critical organ, despite delayed filling and the short half-life of 11C (13). 11C-GMOM did not accumulate in the bladder, but rather in the kidneys, suggesting that the main route of tracer excretion is not through the urinary system and emptying of the bladder will not reduce the dose significantly. The results of the present study are in line with the dosimetry of another methylguanidine derivate, 11C-CNS5161, of which the highest dose was also observed in the lungs and spleen (15).
A whole-body scan (from brain to upper thigh) typically required 11 bed positions, taking about 7.5 min in total. The assumption was made that radiotracer kinetics between first and last bed positions were the same and that the main effect was decay, although this was not the case especially during the early phases of tracer distribution. The resulting uncertainty in organ dose estimates could have been minimized using shorter PET frames for the first whole-body scans. However, organs with high uptake in the first frame were located toward the center of a single bed position (i.e., midframe), and overlap between consecutive bed positions was approximately 50% to maintain a constant axial coverage.
Seven organs were designated source organs after visual inspection of the PET images. Absorbed radiation doses in these organs were low (e.g., 12.7 μGy·MBq−1 in spleen) compared with other 11C-labeled radiotracers (13). The mean absorbed dose in the critical organ of 32 radiotracers tested in humans was shown to be 40 μGy·MBq−1 (range, 11–194 μGy·MBq−1), a factor 3 higher than the spleen dose in the present study. Mean (±SD) effective dose of the 32 radiotracers was 5.3 ± 1.5 μSv·MBq−1, half an SD higher than the 11C-GMOM effective dose.
CONCLUSION
With an effective dose of 4.5 μSv·MBq−1 and relatively low organ doses, 11C-GMOM has a dosimetry profile that allows for serial PET scanning in a single subject.
DISCLOSURE
This study was supported by the Center for Translational Molecular Medicine (LeARN 02N-101) and European Union’s Seventh Framework Programme (FP7/2007-2013), grant agreement no. HEALTH-F2-2011-278850 (INMiND). No other potential conflict of interest relevant to this study was reported.
Footnotes
Published online Feb. 9, 2017.
- © 2017 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication December 6, 2016.
- Accepted for publication January 2, 2017.