Abstract
Patient premedication with carbidopa seems to improve the accuracy of 6-18F-fluoro-3,4-dihydroxy-l-phenylalanine (18F-FDOPA) PET for insulinoma diagnosis. However, the risk of PET false-negative results in the presence of carbidopa is a concern. Consequently, we aimed to evaluate the effect of carbidopa on 18F-FDOPA uptake in insulinoma β-cells and an insulinoma xenograft model in mice. Methods: 18F-FDOPA in vitro accumulation was assessed in the murine β-cell line RIN-m5F. In vivo small-animal PET experiments were performed on tumor-bearing nude mice after subcutaneous injection of RIN-m5F cells. Experiments were conducted with and without carbidopa pretreatment. Results: Incubation of RIN-m5F cells with 80 μM carbidopa did not significantly affect the cellular accumulation of 18F-FDOPA. Tumor xenografts were clearly detectable by small-animal PET in all cases. Insulinoma xenografts in carbidopa-treated mice showed significantly higher 18F-FDOPA uptake than those in nontreated mice. Regardless of carbidopa premedication, the xenografts were characterized by an early increase in 18F-FDOPA uptake and then a progressive reduction over time. Conclusion: Carbidopa did not influence in vitro 18F-FDOPA accumulation in RIN-m5F cells but improved insulinoma imaging in vivo. Our findings increase current knowledge about the 18F-FDOPA uptake profile of RIN-m5F cells and a related xenograft model. To our knowledge, the present work represents the first preclinical research specifically focused on insulinomas, with potential translational implications.
Insulinoma is a rare, usually solitary and benign neuroendocrine tumor (NET). It is characterized by inappropriate and uncontrolled insulin production and secretion, with consequent glycopenic symptoms and potentially lethal hypoglycemia. Early detection of the tumor is crucial, allowing curative treatment by surgical enucleation. The localization of insulinoma remains challenging, and conventional imaging is the first method used for diagnostic investigation (1).
Functional imaging may be of particular interest when the results of conventional imaging are negative or inconclusive (2). Nevertheless, the gold standard examination remains to be defined. Radiolabeled glucagonlike peptide 1 analogs used for conventional scintigraphic studies or PET imaging have shown promising results because glucagonlike peptide 1 receptors are overexpressed in most insulinomas (3). However, these radiopharmaceuticals are not yet commercially available and are used in humans mostly in clinical trials.
6-18F-fluoro-3,4-dihydroxy-l-phenylalanine (18F-FDOPA) is clinically approved and commercially available for PET imaging of insulinomas in many countries. Once internalized by specific cell membrane transporters, 18F-FDOPA is decarboxylated to 18F-dopamine by the aromatic amino acid decarboxylase (AADC) and accumulates in secretory vesicles. Therefore, the expression of AADC has a key role in tumor visualization by 18F-FDOPA PET (4). However, the intense and prolonged radiotracer uptake in the mature exocrine pancreas represents the main drawback of 18F-FDOPA PET and may explain the initial disappointing results for insulinoma detection (5). Furthermore, early PET images and patient premedication with carbidopa may improve the diagnostic accuracy of 18F-FDOPA PET in adult patients with insulinoma-related hyperinsulinemic hypoglycemia (6,7). Carbidopa, an efficient inhibitor of AADC, is capable of drastically reducing physiologic pancreatic uptake, with a consequent increase in the tumor-to-background ratio (8). Moreover, carbidopa leads to better systemic radiotracer availability by reducing the peripheral conversion of 18F-FDOPA to 18F-dopamine and its renal excretion. However, no final consensus about the usefulness of carbidopa premedication before 18F-FDOPA PET in patients with hyperinsulinemic hypoglycemia has been reached because of the potential reduction of tumoral uptake intensity (9). The risk of 18F-FDOPA PET false-negative results could be high for tumors with low intracellular AADC activity (10), which are potentially more sensitive to the inhibitory effect of carbidopa.
The positive impact of carbidopa premedication has been demonstrated in both in vitro and in vivo studies with cell lines of human pancreatic NETs (BON cells) and a related tumor xenograft animal model (11,12). To the best of our knowledge, no preclinical data concerning the influence of carbidopa on 18F-FDOPA uptake in insulinomas are available. Therefore, we aimed to evaluate the effect of carbidopa on 18F-FDOPA uptake in the β-cell line RIN-m5F and a related insulinoma xenograft model developed in nude mice. To our knowledge, the present work represents the first preclinical research study focused on insulinomas, with a potential impact on the diagnostic and therapeutic management of patients in clinical practice.
MATERIALS AND METHODS
Radiotracer Production
Good manufacturing practices–compliant 18F-FDOPA was obtained from Cisbio International (DOPACIS), and electrophilic substitution was performed in accordance with the method reported by de Vries et al. (13). A total activity of 200–240 MBq in 2.2 mL was received and further diluted with 0.9% sterile NaCl to reach an injection volume of 150 μL.
Cell Culture
In vitro experiments were performed with the β-cell line RIN-m5F (CRL-11605; American Type Culture Collection) (14). These cells produce and secrete insulin and produce AADC. Unlike the parental line, they do not produce somatostatin. Positive 18F-FDOPA uptake in cell line RIN-m5F was previously reported (15). Cells were cultured in 75-cm2 flasks containing 20 mL of RPMI 1640 medium without N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid but with glutamine (Invitrogen) (RPMI culture medium). The medium was supplemented with 10% fetal bovine serum (Gibco) and gentamicin (100 μg/mL; Panpharma). Cells were grown at 37°C in a humidified atmosphere containing 5% CO2 and subcultured twice per week.
In Vitro Determination of 18F-FDOPA Accumulation
Flasks were maintained at 37°C and incubated for 1 h either with or without 80 μM carbidopa (INRESA). In another condition, 2 h before the experiment, RPMI culture medium was replaced with an amino-acid-free medium composed of phosphate-buffered saline (0.9 mM CaCl2, 0.5 mM MgCl2, 2.6 mM KCl, 1.47 mM KH2PO4, 138 mM NaCl, 8 mM Na2HPO4; pH 7; Gibco) and glucose (0.75 mM d-glucose) (PBS-Glu medium). Then, 200 kBq of buffered 18F-FDOPA dissolved in 100–150 μL of culture medium were added to each flask. After 5, 30, and 60 min, the culture medium or PBS-Glu medium was removed, and the cells were washed, harvested by trypsin treatment, and suspended in the same type and volume of culture medium (RPMI or PBS-Glu). A 2.5-mL volume of culture cells or of removed medium was transferred to 5-mL counting tubes, and radioactivity was measured in a calibrated γ-counter (Wallac, Perkin-Elmer). For nonspecific tracer adsorption, the protocol was reproduced identically without cells. The viability and number of cells were determined by the trypan blue exclusion technique with a Kova Glastic Slide 10 (Hycor Biomedical).
In a similar experiment with RPMI culture medium, cells were incubated separately with 10 mM 2-aminobicyclo[2.2.1]heptane-2-carboxylic acid (BCH), described as an l-amino acid transporter inhibitor (16), and 10 μM tetrabenazine, described as an inhibitor of the vesicular monoamine transporter (17).
Animals
Twenty female athymic nude mice (24–28 g, 8–10 wk old; Charles River) were used for the entire experimental protocol. To optimize the management of mice with insulinoma xenografts, the carbidopa administration, and the PET imaging protocol, we used 10 of 20 animals (control group) to assess the morphologic evolution of the tumor xenograft (i.e., tumor size) and the related consequences of tumor insulin secretion on animal welfare (i.e., reduction in serum glycemia and the occurrence of lethal hypoglycemia). The remaining 10 mice (experimental group) were dedicated to in vivo small-animal PET investigations of insulinoma xenografts. Animals were kept at constant temperature (22°C) and humidity (40%) in 12-h light–dark cycles and were allowed free access to forage and water until the beginning of each imaging procedure. Animals were housed in individually ventilated cages with high-efficiency particulate air filters in the dedicated facility of the Institut Pluridisciplinaire Hubert Curien (IPHC), Strasbourg, France.
Mice with Tumor Xenografts
A cell suspension of 6 × 106 RIN-m5F cells in 100 μL of phosphate-buffered saline was injected subcutaneously into the right hind leg of mice under inhalation anesthesia (1.5%–2% isoflurane). Tumor width and length were measured (mm) with a caliper to calculate the tumor volume according to the following formula: volume (mm3) = (length × width squared)/2. To evaluate insulin production, we assessed morning serum glycemia levels in blood samples collected from the tail vein of nonfasting mice with xenografts by using a commercial blood glucose monitoring system (Contour TS; Bayer).
18F-FDOPA Small-Animal PET Imaging
Animals were scanned when the tumor reached a minimum dimension of 2.5 × 2.5 mm or a volume of greater than 20 mm3. Mice were divided into 2 groups according to carbidopa pretreatment (i.e., carbidopa-treated or nontreated mice). Carbidopa was administered orally via a gavage needle at a dose of 20 μg in 100 μL of NaCl 1 h before the intravenous injection of 18F-FDOPA. In all animals, 18F-FDOPA was injected through the jugular vein, and PET images were acquired on a small-animal PET scanner (Iris; Inviscan) with a spatial resolution of less than 1.4 mm in the reconstructed PET images. As a preventive measure, 300 μL of 20% glucose solution were administrated orally via a gavage needle 30 min before the imaging procedure. For small-animal PET imaging, ketamine–xylazine anesthesia was induced by an intraperitoneal injection of ketamine (50 mg/kg) and xylazine (6 mg/kg) in a volume of 100 μL/30 g of body weight and was maintained with 1.5% isoflurane in medical air with a calibrated vaporizer for the entire procedure. Dynamic 3-dimensional PET acquisitions were performed for 20 min starting 2 min after the 18F-FDOPA injection. During PET acquisition, animal temperature and respiration rate were monitored to assess the depth of anesthesia. Throughout the investigation, mice were kept at a constant temperature of 37°C.
Acquired list-mode PET data were binned into 4 frames of 5 min each and 20 frames of 1 min each. Data were reconstructed into a 201 × 201 × 120 three-dimensional volume by use of the iterative 3-dimensional ordered-subset expectation maximization algorithm with 6 iterations and 8 subsets. The voxel size was equal to 0.42 mm in the transverse plane and 0.85 mm in the axial direction. The calibration factor was included in the normalization file and applied during the reconstruction process. PET data were fully corrected for random coincidences, radioactive decay, and dead time. No attenuation or scatter corrections were applied.
18F-FDOPA PET image analysis was performed with the AMIDE software package (http://amide.sourceforge.net/). The results of small-animal PET images were qualitatively interpreted as positive or negative. A focal nonphysiologic increase in radiotracer uptake in the area of tumor development was considered to be a positive PET result. For a semiquantitative assessment of tumoral 18F-FDOPA uptake, an elliptic volume of interest (VOI) was drawn around the tumor on the image from the first 5 min of the PET acquisition. Tumoral VOI activity was first corrected with the mean voxel value measured in the contralateral thigh muscle. Afterward, only voxels with values higher than 40% of the maximum voxel value in the tumor were considered for further analysis. Elliptic VOIs were also drawn around the pancreas, liver, and mediastinum. Finally, time–activity curves were extracted from VOIs for the whole dynamic PET acquisition. SUVs were calculated from the mean voxel value within the VOIs. SUVs were recorded and used for generating time–activity curves.
All animal experiments were performed in accordance with European Institutes of Health Guidelines regarding the care and use of animals for experimental procedures and were approved by the Alsace Regional Ethics Committee for Animal Experimentation (approval identification: APAFIS#2944).
Data Analysis
Differences between various groups were tested for statistical significance with nonparametric tests such as the Friedmann test for repeated measures, with adapted post hoc tests, and the Mann–Whitney U test for independent samples, as appropriate. The justification for such statistics was based on the small number of samples in some conditions. For convenience, the results are represented as the mean ± SE of the mean. A P value of less than 0.05 was considered to be significant. Statistical analysis was performed with the SPSS Statistics (IBM) software package.
RESULTS
In Vitro Determination of 18F-FDOPA Accumulation
Control Condition
The accumulation of 18F-FDOPA in cells incubated in RPMI culture medium was almost constant over 60 min. Only a slight increase in 18F-FDOPA uptake, ranging from 2.9%/106 cells at 5 min to 4.5%/106 cells at 60 min, was observed. In the control condition with amino acid–free PBS-Glu medium, cellular 18F-FDOPA uptake decreased by about 3.7% over time, with values ranging from 6.8%/106 cells at 5 min to 3.1%/106 cells at 60 min (Fig. 1A). The highest amount of radiotracer uptake was observed at the earliest time point (5 min) and was more than twice as high as that in the RPMI culture medium condition. No statistically significant differences were observed within each culture condition over the 3 tested time points with standard culture medium or with amino acid–free PBS-Glu medium (P = 0.395 and P = 0.135, respectively). Nonetheless, statistical significance was observed between the 2 culture media in the control condition at the first (P < 0.01) and last (P < 0.05) time points.
Accumulation of 18F-FDOPA in RIN-5mF cells. Tracer accumulation was measured in classical RPMI culture medium and amino acid–free medium (PBS-Glu medium). Values represent mean percentage of uptake for 106 cells ± SE of mean. (A) Effect of carbidopa (80 μM) on 18F-FDOPA uptake in RPMI culture medium (n = 12 for each condition) and in PBS-Glu medium (n = 4 for each condition). *P < 0.05 (Mann–Whitney U test). (B) Effect of BCH (10 mM; n = 4) and tetrabenazine (TBZ; 10 μM; n = 2) on 18F-FDOPA uptake in RPMI culture medium. #P < 0.05 (Mann–Whitney U test).
Inhibition Experiments
Incubation of cells with 80 μM carbidopa did not significantly affect 18F-FDOPA accumulation in RIN-m5F cells. As in the control condition, 2 different dynamic 18F-FDOPA uptake patterns were observed according to the culture medium. In the standard condition (RPMI culture medium), cells showed quite stable 18F-FDOPA uptake over time. In PBS-Glu medium, 18F-FDOPA uptake was highest at the earliest time point (5 min) and decreased over time, with values ranging from 4.6%/106 cells at 5 min to 3.3%/106 cells at 60 min (Fig. 1A). Overall, no statistically significant differences in 18F-FDOPA uptake were observed between carbidopa-treated and nontreated cells at the 3 tested time points of incubation with RPMI culture medium or with PBS-Glu medium. BCH, a selective inhibitor of the l-amino acid transporter, drastically reduced the tracer uptake at all studied time points in a statistically significant manner (P < 0.05). Tetrabenazine led to a similar constant decrease in 18F-FDOPA uptake over the studied period (P < 0.05) (Fig. 1B).
Mice with Tumor Xenografts and 18F-FDOPA Small-Animal PET Imaging
Control Group
The subcutaneous tumor xenograft was visible about 2 wk after cellular inoculation of the RIN-m5F cell suspension, reaching a volume of at least 20 mm3 about 3 wk after cellular injection. Serial measurements of morning serum glycemia levels obtained before and after the subcutaneous cellular injection highlighted a progressive decrease in the blood glucose concentration in mice, confirming the secreting character of the insulinoma xenograft (Fig. 2A). Consequently, water was replaced with a 20% glucose solution 15 d after the subcutaneous injection of the RIN-5mF cell suspension in an attempt to limit the severity of glycopenic symptoms (decrease in spontaneous activity followed by no activity, tremor, tachypnea, or hypopnea) and to reduce the occurrence of lethal hypoglycemia in animals. No significant correlation was observed between the glycemia value and the insulinoma xenograft size. Pathologic examination of tumor xenografts was performed in 2 mice that died after a hypoglycemic crisis (Fig. 2B). Analysis revealed the presence of epithelioid and spindle cells staining for insulin (Figs. 2C and 2D).
Functional and pathologic characterization of insulinoma xenografts. (A) Morning serum glycemia levels in blood samples collected from 10 nonfasting mice with xenografts. Dashed line represents introduction of 20% glucose solution instead of water. (B) Insulinoma xenograft of 1.4 × 1.3 mm (arrow) developed 15 d after subcutaneous injection of 6 × 106 RIN-5mF cells. Insulinoma-bearing mice died after hypoglycemic crisis (serum glycemia, 18 mg/dL). (C) Histologic analysis of RIN-5mF xenograft tumor showing epithelioid and spindle cells (hematoxylin–eosin stain, ×400). (D) Immunohistochemical analysis of RIN-5mF xenograft tumor showing insulin-positive immunostaining.
Experimental Group
Despite the use of a 20% glucose solution instead of water, 4 animals died because of a hypoglycemic crisis within 12 d after inoculation of the RIN-m5F cell suspension and before the appearance of a measurable insulinoma xenograft. Hence, 6 mice underwent 18F-FDOPA small-animal PET investigations. Two experimental groups consisted of 3 mice each. Animals in the first group were pretreated with 20 μg of carbidopa about 1 h before the intravenous injection of 18F-FDOPA (5.1 ± 0.9 MBq in 150 μL). Mice in the second group received no premedication before intravenous 18F-FDOPA administration (7.5 ± 1.2 MBq in 150 μL). Small-animal PET studies were performed 3–4 wk after the inoculation of the RIN-m5F cell suspension, and the tumor xenograft volume ranged from 20 to 159 mm3. No significant difference in tumor size was observed between carbidopa-treated (77 ± 42 mm3) and nontreated (53 ± 22 mm3) mice, and the level of glycemia was less than 60 mg/dL in all cases. Glycemia levels and insulinoma sizes were not significantly correlated.
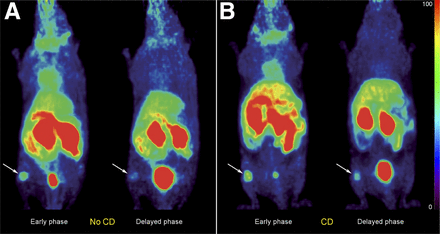
The tumor xenograft was clearly detectable in all mice regardless of carbidopa premedication (Fig. 3). The mean time–activity curves resulting from the tumor xenografts in both carbidopa-treated mice and nontreated mice are shown in Figure 4. The dynamic profiles of tumoral 18F-FDOPA uptake in carbidopa-treated and nontreated mice were similar. The insulinoma xenograft was characterized by an early increase in 18F-FDOPA uptake after radiotracer injection, reaching a peak intensity about 5 min after radiotracer injection. Then, 18F-FDOPA uptake progressively decreased over time until the end of the dynamic PET acquisition (∼35% reduction in the initial SUV). The tumor remained detectable during the whole PET examination in carbidopa-treated and nontreated animals (Fig. 3).
Representative anterior view of 18F-FDOPA PET maximum-intensity-projection images obtained without (A) and with (B) carbidopa (CD) premedication of 2 nude mice with subcutaneous insulinoma xenografts of 38 and 52 mm3, respectively, in right hind legs (arrows). Images were reconstructed from summed time frames of 0–5 min (early phase) and 15–20 min (delayed phase) after intravenous 18F-FDOPA injection. Insulinomas were detected regardless of carbidopa treatment. Moreover, tumor SUV in treated mice was higher than that in nontreated mice for both early and delayed PET acquisitions (SUVs: no CD, early phase, 0.94; no CD, delayed phase, 0.46; CD, early phase, 1.46; CD, delayed phase, 1.03).
18F-FDOPA PET time–activity curves for insulinoma xenografts according to carbidopa (CD) premedication (no CD vs. CD). Tumors from CD-treated mice showed significantly higher 18F-FDOPA uptake over whole PET acquisition than tumors from nontreated mice (P < 0.05). SUVs are reported as median and interquartile range.
There was no negative influence of carbidopa on tumoral 18F-FDOPA uptake in animals pretreated with carbidopa. On the contrary, insulinoma xenografts from carbidopa-treated mice showed significantly higher 18F-FDOPA uptake (P < 0.05) over the whole PET acquisition than tumors from nontreated mice (Fig. 4).
Visually, carbidopa-treated mice showed increased 18F-FDOPA uptake in abdominal organs, such as the pancreas and liver (Fig. 5); this finding could be explained by reduced peripheral radiotracer degradation by carbidopa and consequent increased availability for both the tumor and abdominal organs. Statistical significance was not reached, probably because of the small number of animals analyzed.
Biodistribution of 18F-FDOPA in mice bearing insulinoma xenografts according to carbidopa (CD) premedication (no CD vs. CD). SUVs (mean ± SE of mean) were obtained from summed time frames of 0–5 min (early phase) and 15–20 min (delayed phase) in dynamic PET study after 18F-FDOPA injection. 18F-FDOPA degradation in peripheral organs was inhibited by CD, with consequent increase in radiotracer availability and accumulation in abdominal organs. Statistical significance was not reached, probably because of small number of animals analyzed.
DISCUSSION
To the best of our knowledge, this is the first study evaluating the effect of carbidopa on 18F-FDOPA uptake in β-cells and the related xenograft model in mice. The most important conclusions were that carbidopa did not induce a significant difference in 18F-FDOPA uptake intensity in the β-cell line RIN-5mF and that no negative influence on PET detection of insulinoma xenografts in mice premedicated with carbidopa was observed. On the contrary, carbidopa treatment significantly improved 18F-FDOPA uptake in RIN-m5F tumor xenografts.
The accumulation of 18F-FDOPA in RIN-5mF cells was sensitive to the presence of amino acids within the culture medium. In RPMI culture medium, 18F-FDOPA cellular uptake was almost constant over 60 min. On the other hand, an important increase in 18F-FDOPA uptake at the earliest time point (5 min) and then a progressive decrease were observed in amino acid–free medium. Early competition for cellular intake between 18F-FDOPA and other amino acids present in RPMI culture medium could support this observation.
No statistically significant effect on the uptake of 18F-FDOPA in RIN-5mF cells over time was observed, regardless of the culture medium. This finding indicates that the intracellular decarboxylation process in RIN-5mF cells was not considerably affected by the pharmacologic inhibition by carbidopa. The upregulation of AADC activity in NET cells supports this finding (10,17).
To our knowledge, there are no previously reported experimental data on the effect of carbidopa on the RIN-5mF cell line to allow further comparison. However, similar investigations were previously performed with BON cells. According to Neels et al. (11), the incubation of BON cells with carbidopa did not affect the accumulation of 18F-FDOPA in full culture medium or PBS-Glu medium. Moreover, the accumulation of 18F-FDOPA was more rapid and reached a much higher level in amino acid–free PBS-Glu medium than in standard culture medium. These results were partially confirmed by Kuik et al. (12), who recently reported a significant decrease in 18F-FDOPA accumulation after carbidopa treatment only at late time points under conditions of amino acid depletion.
The experiment performed under BCH inhibition underlines the importance of the amino acid metabolic pathway in RIN-5mF cells. The deregulation of amino acid intake by specific pharmacologic agents targeting the l-amino acid transporter may emerge as an interesting therapeutic option, as previously observed during in vitro and in vivo studies of gliomas (18).
Tetrabenazine led to a similar but less extensive decrease in 18F-FDOPA uptake than BCH. In this experimental condition, 18F-dopamine was not transported into cellular granules by vesicular monoamine transporters and thus was potentially cleared from cells.
The advantage of carbidopa administration has been suggested in mice with NET xenografts from BON cells and in patients with pancreatic NETs (11,19). Carbidopa improved image interpretation for 18F-FDOPA PET by increasing tumoral uptake and decreasing physiologic pancreatic accumulation. We observed similar effects in nude mice bearing RIN-m5F insulinoma xenografts. Accordingly, under carbidopa inhibition, 18F-FDOPA uptake was significantly higher than in nontreated mice.
In the present study, the carbidopa administration protocol was optimized before PET imaging. The combination of repeated anesthesia and the physical trauma from intraperitoneal injections of carbidopa was poorly tolerated in weakened mice with insulinoma-related chronic hypoglycemia. For this reason, in the experimental group, carbidopa was administrated orally 1 h before small-animal PET imaging.
Overall, the absence of tumoral inhibition, the low physiologic pancreatic uptake, and the increase in 18F-FDOPA availability could be considered the underlying effects of carbidopa premedication even in the RIN-m5F insulinoma xenograft model.
Regardless of carbidopa premedication, RIN-m5F insulinoma xenografts showed the highest uptake of 18F-FDOPA within 5 min after radiotracer injection and then a continuous decrease until the end of the studied period. This kinetic pattern was close to that observed in vitro with a culture medium supplemented with d-glucose (PBS-Glu medium). The oral overload of glucose before small-animal PET imaging may help explain the results of the in vivo experiments.
We recently reported insulinomas showing pathologic 18F-FDOPA uptake exclusively on early images in patients who underwent carbidopa-assisted PET/CT (6,7). In these cases, the influence of carbidopa on rapid tumoral washout could not be completely ruled out. In our preclinical model, the dynamic profiles of 18F-FDOPA uptake in both carbidopa-treated mice and nontreated mice were similar. Hence, carbidopa premedication did not seem to be directly related to the dynamic pattern of 18F-FDOPA uptake. Kauhanen et al. (9) reported the disappearance of 18F-FDOPA focal pancreatic uptake after carbidopa administration in 2 patients with histologically proven insulinoma and β-cell hyperplasia. In these cases, the negative PET result was more likely related to the nonoptimized PET acquisition protocol than to carbidopa-related tumoral inhibition. Parallel 18F-FDOPA catabolic pathways through, for example, catechol O-methyltransferase warrant further investigation.
Significant differences between mice and humans in terms of methodologic issues regarding carbidopa dose, interspecies carbidopa metabolism, fasting conditions, and glucose charge before PET should be taken into consideration. Moreover, the growth pattern of our tumor xenografts was more rapid than that of human insulinomas, which are typically benign. Thus, caution in interpreting preclinical data is required. The malignant potential of our xenografts may have been amplified by the athymic status of the host (nude mice) and by the several cellular subcultures before subcutaneous implantation. However, 2 animals that underwent 18F-FDG small-animal PET showed no significant radiotracer uptake in xenografts (data not shown). Such a finding is not typical for malignant tumors, including malignant insulinomas. Discordant results between in vivo and in vitro experiments have also been reported for evaluation of the effects of carbidopa on BON cells and related xenograft tumors (11).
Collantes et al. (15) reported the results of 18F-FDOPA PET of 2 nude mice with RIN-m5F insulinoma xenografts. The PET results for both mice were positive, showing 18F-FDOPA uptake about 60 min after intravenous 18F-FDOPA injection. Neither dynamic PET acquisition nor carbidopa premedication was performed to allow further comparison with our results. Moreover, this group reported differential expression of type 2 vesicular monoamine transporters in RIN cells between in vitro and in vivo experiments; consequently, deductions about translation must be made with caution.
The small number of mice that underwent 18F-FDOPA PET may represent a limitation of the present study. Moreover, no animal was examined twice, according to an intraindividual analysis, with and without carbidopa premedication. However, the high level of care required for the tumoral xenograft model, which was characterized by elevated mortality due to severe hypoglycemia before the appearance of measurable insulinoma xenografts, justified our study protocol.
CONCLUSION
Carbidopa did not influence in vitro 18F-FDOPA accumulation in RIN-m5F cells but improved tumor imaging in vivo. Our findings increase current knowledge about the 18F-FDOPA uptake profile of RIN-m5F cells and a related xenograft model. To our knowledge, the present work represents the first preclinical research specifically focused on insulinomas, with potential translational implications.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank Gerlinde Averous, MD, for pathological examinations of RIN-m5F insulinoma xenografts; Patrice Laquerriere, PhD, for assistance with statistics; and Lionel Thomas, PhD, and Bruno Jessel for technical support in animal care. Cisbio International–IBA Molecular is kindly acknowledged for providing 18F-FDOPA (DOPACIS).
Footnotes
Published online Sep. 8, 2016.
- © 2017 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication July 4, 2016.
- Accepted for publication August 10, 2016.