Abstract
The aim of this study was to develop a method for the quantification of hepatobiliary uptake and secretion of conjugated bile acids with PET and the 11C-labeled conjugated bile acid analog [N-methyl-11C]cholylsarcosine (11C-CSar). Methods: Six pigs (13 experiments) underwent dynamic 11C-CSar PET of the liver with simultaneous measurements of hepatic blood perfusion and 11C-CSar concentrations in arterial, portal, and hepatic venous blood. In 3 pigs (7 experiments), bile was collected from a catheter in the common hepatic duct. PET data were analyzed with a 2-tissue compartmental model with calculation of rate constants for the transport of 11C-CSar among blood, hepatocytes, and intra- and extrahepatic bile ducts. PET results were validated against invasive blood and bile measurements. Results: The directly measured rate of secretion of 11C-CSar into bile was equal to the rate of removal from blood at steady state. Accordingly, hepatocytes did not accumulate bile acids but simply facilitated the transport of bile acids from blood to bile against a measured concentration gradient of 4,000. The rate constant for the secretion of 11C-CSar from hepatocytes into bile in experiments with a catheter in the common hepatic duct was 25% of that in experiments without a catheter (P < 0.05); we interpreted this result to be mild cholestasis caused by the catheter. The catheter caused an increased backflux of 11C-CSar from hepatocytes to blood, and hepatic blood flow was 25% higher than in experiments without the catheter. The capacity for the overall transport of 11C-CSar from blood to bile, as quantified by intrinsic clearance, was significantly lower in experiments with the catheter than in those without the catheter (P < 0.001). PET and blood measurements correlated significantly (P < 0.05). Conclusion: The in vivo kinetics of hepatobiliary secretion of conjugated bile acids can now be determined by dynamic 11C-CSar PET.
- hepatic excretory function
- in vivo liver kinetics
- bile salt export pump
- intrahepatic cholestasis
- functional molecular imaging
Conjugated bile acids, the major constituents of bile, are essential for efficient intestinal absorption of dietary lipids, including lipophilic vitamins and drugs (1–3). The major part of the bile acid pool in humans circulates enterohepatically, and hepatic de novo synthesis constitutes only about 5% of bile acid secretion (1–3). Hepatocellular uptake of conjugated bile acids from sinusoidal blood is primarily mediated by the Na+-taurocholate–cotransporting polypeptide (NTCP; SLC10A1) and canalicular secretion from hepatocytes into bile by the bile salt export pump (BSEP; ABCB11) (3–6). BSEP is impaired in several cholestatic disorders, such as progressive familial intrahepatic cholestasis type 2, drug-induced cholestasis, primary biliary cholangitis, and primary sclerosing cholangitis; all of these conditions may lead to the accumulation of toxic levels of bile acids in hepatocytes (3–6). However, studying hepatobiliary uptake and secretion of bile acids in vivo is difficult. Isotopically labeled bile acids have been used to study bile acid uptake and secretion in the isolated perfused rat liver (7,8) and conscious dogs (9), and sandwich cultures have been used in attempts to predict the in vivo kinetics of bile acid turnover (10–12). Clinical methods, such as 99mTc-mebrofenin SPECT (13) and novel PET tracers for hepatic drug transporters (14), have been used to assess different aspects of hepatic excretory function but not specific bile acid transporters.
The radiolabeled conjugated bile acid analog [N-methyl-11C]cholylsarcosine (11C-CSar) is a novel tracer for PET of hepatobiliary secretion (15). Proof-of-concept studies in pigs validated that 11C-CSar is secreted into bile and circulates enterohepatically without being metabolized; these properties simplified the analysis of PET data by reducing the number of compartments (14,16). The purpose of the present pig study was to determine the in vivo kinetics of hepatobiliary secretion of 11C-CSar by dynamic PET of the liver for the quantification of individual transport steps and bile flow. The findings were validated against invasive blood and bile measurements.
MATERIALS AND METHODS
Experimental Setup
Six anesthetized pigs (3–4 mo old, female, Danish Landrace and Yorkshire crossbreed; body weight, 39–42 kg) underwent 1–3 successive PET experiments (13 experiments in total) with 11C-CSar administered as a continuous intravenous infusion initiated by a priming bolus. At least 6 radioactive half-lives (i.e., 2 h) were allowed to pass between successive 11C-CSar administrations. During the PET recording, blood was collected from a femoral artery, the portal vein, and a hepatic vein for measurements of the concentrations of 11C-CSar in blood. Hepatic blood flow was measured continuously, and in 3 pigs (7 experiments), bile was collected for measurements of the bile flow rate and biliary concentrations of 11C-CSar.
The study was approved by the Danish Animal Experiments Inspectorate and performed in accordance with the Danish Animal Experimentation Act and European conventions for the protection of vertebrate animals used for experimental and other purposes.
Animal Preparation
Animals were sedated with an intramuscular injection of midazolam and S-ketamine; anesthetized with an infusion of midazolam, S-ketamine, and propofol; mechanically ventilated; and kept physiologically stable (17).
For intravenous infusions, Avanti catheters (Cordis) were inserted into the femoral veins. For blood sampling, an Avanti catheter was placed in a femoral artery, and a Cournand 6F catheter (Cook) was placed in a hepatic vein via the right jugular vein. The liver was accessed through a 30-cm-long subcostal incision below the right curvature, and a 5.3F catheter (Cook) was inserted directly into the portal vein for blood sampling. Ultrasound transit-time flow meter probes (VeriQ; Medistim) were placed around the portal vein and hepatic arteries for continuous recording of blood flow rates. In 3 pigs, a modified Silkolatex Kehr T-tube catheter (Willy Rusch GmbH) was placed directly in the common hepatic duct (CHD) for the collection of bile.
Total hepatic blood flow rate, Fblood (mL blood/min), was calculated as FHA + FPV, where FHA is the hepatic arterial blood flow rate (mL blood/min) and FPV is the blood flow rate in the portal vein (mL blood/min). Fblood deviated less than 5% from the mean value during each PET experiment, and individual mean values of Fblood were used.
After the last experiment, the pigs were given an overdose of phenobarbital (4 g), and the liver was removed and weighed. The liver volume, Vliver (mL liver tissue), was calculated with a liver density of 1.07 g/mL liver tissue (18) and used to calculate hepatic blood perfusion, Q (mL blood/min/mL liver tissue), as Fblood/Vliver.
PET Recording
Pigs were placed in the supine position in a Biograph 64 Truepoint PET/CT camera (Siemens AG). A topogram of the upper abdomen was used to position the liver and bile ducts within the 21.6-cm transaxial field of view, and a low-dose CT scan (50 mAs [effective] with CAREDose 4D [Siemens]; 120 kV; pitch, 0.8; slice thickness, 5 mm) was used for attenuation correction of PET emission data and anatomy. 11C-CSar was produced as described elsewhere (15) and administered intravenously as a priming bolus of 100 MBq (range, 50‒145 MBq) during the initial 20–25 s of the dynamic 60-min PET recording (list mode) and then as a continuous infusion of 7.2 MBq/min (range, 4.0‒10.5 MBq/min). PET data were reconstructed by use of attenuation-weighted ordered-subset expectation maximization with resolution recovery, a 336 × 336 matrix, a voxel size of 2 × 2 × 2 mm, 4 iterations, 21 subsets, a 2-mm gaussian filter, separate prompts/randoms, and a central spatial resolution of 4.2 mm (full width at half maximum). The time frame structure was 20 × 5 s, 15 × 10 s, 3 × 30 s, 4 × 1 min, 1 × 3 min, and 10 × 5 min, and data were corrected for radioactive decay back to the start of the PET recording.
Using fused PET/CT images, we drew regions of interest on adjacent slices of liver tissue, with the exclusion of large blood vessels and bile ducts and with a distance of at least 3 cm to the gallbladder. The regions of interest were summed to a volume of interest from which the time course for the concentration of 11C-CSar in liver tissue, Cliver(t) (kBq/cm3 liver tissue), was generated.
Blood and Bile Samples
During the PET recording, 0.5-mL blood samples were collected at time points 12 × 5 s, 6 × 10 s, 6 × 30 s, 5 × 1 min, and 5 × 10 min from the femoral artery, portal vein, and hepatic vein for measurements of concentrations of 11C-CSar in blood (Packard well counter). Measurements were corrected for radioactive decay back to the start of the PET recording, and time courses for the concentrations of 11C-CSar in blood (kBq/mL blood) were generated for the hepatic artery, CHA(t) (identical to that measured in the femoral artery), the portal vein, CPV(t), and the hepatic vein, Cout(t). The time course for the flow-weighted mixed-inlet concentration of 11C-CSar in the liver, Cin(t) (kBq/mL blood), was calculated (19) as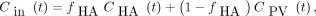 Eq. 1where fHA is the arterial flow fraction calculated as FHA/Fblood; it was, on average, 0.26 (range, 0.18–0.39), with no systematic changes during the experiments.
Eq. 1where fHA is the arterial flow fraction calculated as FHA/Fblood; it was, on average, 0.26 (range, 0.18–0.39), with no systematic changes during the experiments.
Bile was collected by being allowed to flow continuously from the CHD catheter into test tubes, which were weighed before and after sampling. The bile flow rate, Fbile (mL bile/min), calculated with a measured bile density of 1.0 g/mL bile, deviated less than 5% from the mean value during each experiment, and individual mean values of Fbile were used. Measured concentrations of 11C-CSar in bile (kBq/mL bile) were corrected for radioactive decay back to the start of the PET recording and a sampling delay of 6 min, yielding time courses for the concentrations of 11C-CSar in bile, Cbile(t).
Calculations from PET Measurements
PET data were analyzed by fitting the kinetic model of hepatobiliary uptake and secretion (Fig. 1) to the data. The equations for this model are Eq. 2a
Eq. 2a Eq. 2b
Eq. 2b Eq. 2cThe model parameters Kmem, kbackflux, kBSEP, and kbile were estimated by nonlinear least-squares minimization with software designed in-house (www.liver.dk/ifit.html) and with Cliver(t) as the output, Cin(t) as the input, and Vblood set to 0.2 mL blood/cm3 liver tissue. Cbile(t) was measured in experiments with the CHD catheter but was not used to estimate the model parameters.
Eq. 2cThe model parameters Kmem, kbackflux, kBSEP, and kbile were estimated by nonlinear least-squares minimization with software designed in-house (www.liver.dk/ifit.html) and with Cliver(t) as the output, Cin(t) as the input, and Vblood set to 0.2 mL blood/cm3 liver tissue. Cbile(t) was measured in experiments with the CHD catheter but was not used to estimate the model parameters.
Kinetic model fitted to PET data. Liver VOI comprises 3 separate compartments: blood, hepatocytes, and intrahepatic bile volume. Exchange of 11C-CSar among compartments is described by unidirectional removal of 11C-CSar from blood to hepatocytes by clearance Kmem (mL blood/min/mL liver tissue), backflux of tracer from hepatocytes to blood by rate constant kbackflux (min−1), secretion from hepatocytes into bile canaliculi via BSEP by rate constant kBSEP (min−1), and 11C-CSar in bile flowing out of liver volume of interest by rate constant kbile (min−1).
The hepatic systemic clearance of 11C-CSar, Ksys (mL blood/min/mL liver tissue), was calculated (20) as Eq. 3In some cases, the analysis yielded Kmem values higher than Q, an innate problem with compartmental analysis of PET data (21); in these cases, Kmem was calculated as QE0 (unidirectional extraction fraction) in Equation 3 (22).
Eq. 3In some cases, the analysis yielded Kmem values higher than Q, an innate problem with compartmental analysis of PET data (21); in these cases, Kmem was calculated as QE0 (unidirectional extraction fraction) in Equation 3 (22).
The hepatic intrinsic clearance of 11C-CSar, Kint (mL blood/min/mL liver tissue), which is not dependent on flow, was calculated (23) as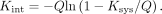 Eq. 4
Eq. 4
Calculations from Blood and Bile Measurements
The time course for the hepatic extraction fraction of 11C-CSar from blood, E(t), was calculated as Eq. 5
Eq. 5
Data from the first minute, when backflux can be ignored (24), were used to calculate the unidirectional extraction fraction of 11C-CSar, E0, from blood to hepatocytes across the hepatocyte sinusoidal membrane; to adjust for non–steady state, Cout was corrected for a mean transit time of 23 s. Steady-state blood concentrations of Cin and Cout at 40–50 min were used to calculate the hepatic steady-state extraction fraction of 11C-CSar, Ess.
The non–flow-dependent permeability–surface area product, PSmem (mL blood/min/mL liver tissue), which is the unidirectional intrinsic clearance of the hepatocyte sinusoidal membrane for 11C-CSar, was calculated (24) as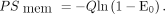 Eq. 6
Eq. 6
The steady-state hepatic rate of removal of 11C-CSar from blood, vss (kBq/min), was calculated for the whole liver as Eq. 7
Eq. 7
The non–flow-dependent hepatic intrinsic clearance of 11C-CSar from blood into bile, Clint (mL blood/min/mL liver tissue), was calculated (23,24) as Eq. 8
Eq. 8
The flow-dependent hepatic systemic clearance of 11C-CSar, Clsys (mL blood/min/mL liver tissue), was calculated as Eq. 9
Eq. 9
The concentration of 11C-CSar in bile at 50 min after the start of the infusion of 11C-CSar, Cbile (kBq/mL bile), was used to calculate the steady-state rate of secretion of 11C-CSar, vbile (kBq/min), as Eq. 10
Eq. 10
The fractional intrahepatic bile volume, Vbile (mL bile/mL liver tissue), was calculated as Eq. 11
Eq. 11
Using the mean Vbile value from experiments with the CHD catheter and individual values for kbile and Vliver from each experiment, we calculated Fbile in pig experiments without the CHD catheter as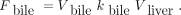 Eq. 12
Eq. 12
Statistical Analysis
Data are reported as means and 95% confidence intervals (CIs) or as means and ranges. Individually measured data were weighted with the inverse squared SEE for the calculation of group mean values. The Student t test was used to test differences between pigs with the CHD catheter and pigs without the CHD catheter; a P value of less than 0.05 was considered to indicate statistical significance.
RESULTS
Figure 2 shows examples of the time courses for 11C-CSar concentrations in blood, liver tissue, and bile in experiments without the catheter in the CHD and experiments with the catheter in the CHD. In all experiments, Cin(t) and Cout(t) became constant after 10‒20 min. Cliver(t) increased rapidly in all experiments. However, steady levels of Cliver(t) were reached in only half of the experiments with the CHD catheter, whereas steady levels were reached within 20 min in two-thirds of the experiments without the CHD catheter. These findings are in agreement with a cholestatic effect that was caused by the CHD catheter and that primarily affected hepatocyte secretion of 11C-CSar into bile, not uptake from blood. Interestingly, Cbile(t) had the same shape as the corresponding Cliver(t), but the concentration in bile was, on average, 200 times higher (Fig. 2C).
(A and B) Time courses of 11C-CSar concentrations in flow-weighted mixed-inlet blood (Cin; red), hepatic venous blood (Cout; blue), and liver tissue (Cliver; black) in experiment without catheter in CHD (A) and experiment with catheter in CHD (B). (C) Time course of 11C-CSar concentration in bile samples (Cbile; green) in same experiment as in B.
The values of the kinetic parameters estimated from PET data are shown in Table 1. Mean kBSEP was many times higher than mean kbackflux, illustrating that secretion from hepatocytes into bile was much more efficient and physiologically significant than backflux. The CHD catheter caused a significant decrease in both kBSEP and Kint, an insignificant decrease in kbile, and a significant increase in kbackflux. These data indicate a minor cholestatic effect of the CHD catheter (reduced kbile) that led to reduced secretion from hepatocytes into bile (kBSEP) which, in turn, led to increased backflux of 11C-CSar from hepatocytes to blood (kbackflux). In accordance with these findings, Kint was significantly higher in experiments without the CHD catheter than in experiments with the CHD catheter. Ksys was unaffected by the CHD catheter; this result can be explained by an increase in Q (discussed later in article) because Ksys is determined by flow.
Hepatobiliary Secretion Kinetics Calculated for 11C-CSar from PET Measurements
Mean steady-state Cbile was 22,000 kBq/mL bile (range, 8,000–38,000 kBq/mL bile); this value was, on average, 4,000 times (range, 2,400–4,700 times) higher than the corresponding Cin. Mean measured Fbile was 0.27 mL bile/min (95% CI, 0.21–0.33 mL bile/min). The fractional intrahepatic bile volume, Vbile, was, on average, 2.1 mL bile/L liver tissue (range, 1.21–7.52 mL bile/L liver tissue). In studies without the CHD catheter, calculated Fbile was, on average, 0.45 mL bile/min (range, 0.37–0.52 mL bile/min) (P < 0.05 in comparison with measured values in experiments with the CHD catheter).
Values for hepatic blood perfusion and kinetic parameters for hepatic uptake from blood and biliary secretion of 11C-CSar calculated from blood measurements are shown in Table 2. Interestingly, Q was, on average, 25% higher in experiments with the CHD catheter than in experiments without the CHD catheter (P < 0.05); this result was caused by an increase in portal vein blood flow with no change in hepatic arterial blood flow.
Hepatobiliary Secretion Kinetics Calculated for 11C-CSar from Blood Measurements
E0 was lower in experiments with the CHD catheter than in experiments without the CHD catheter but was still close to unity; this result indicates high unidirectional transport of 11C-CSar from blood to hepatocytes in both groups of animals. Because E0 is dependent on flow, the small but statistically significant difference between E0 in the 2 groups of animals can be explained by the difference in Q (22,23)—an explanation confirmed by the unaffected, non–flow-dependent PSmem values. Accordingly, the transport capacity for 11C-CSar across the hepatocyte sinusoidal membrane was unaffected by the CHD catheter.
The capacity for the overall transport of 11C-CSar from blood to bile, as quantified by the intrinsic clearance, Clint (Table 2), was significantly lower in pigs with the CHD catheter than in pigs without the CHD catheter (P < 0.001). Furthermore, Ess was significantly lower than E0 in pigs with the CHD catheter (P < 0.05), whereas it was not statistically different from E0 in experiments without the CHD catheter (Table 2). These results show that the reduced secretion of 11C-CSar in experiments with the CHD catheter caused an increased backflux of 11C-CSar from hepatocytes to blood—as also seen for the PET parameters.
The correlation between Clsys from blood data and Ksys from PET data and the correlation between Clint and Kint were both excellent (r2, 0.7; P < 0.05).
DISCUSSION
The main result of the present study is that it is now possible to quantify the individual steps in hepatic uptake and biliary secretion of conjugated bile acids in vivo with dynamic 11C-CSar PET. The method was even able to quantify changes in kinetic parameters caused by the acute, albeit mild, cholestasis induced by the CHD catheter. The finding that the steady-state rate of secretion of 11C-CSar was equal to the rate of removal from blood (Fig. 3) shows that hepatocytes did not accumulate 11C-CSar during the infusion of 11C-CSar but simply facilitated the transport of bile acids from blood to bile against a measured concentration gradient of 4,000.
Relationship between steady-state hepatic rate of removal of 11C-CSar, vss (Eq. 7), calculated from blood measurements, and secretion measured directly from bile sampling, vbile (Eq. 10), in experiments with catheter in CHD. Line of identity is shown. Identical symbols represent repeated measurements in same pig.
The steady-state extraction fraction of 11C-CSar from blood, Ess, was lower than E0 in experiments with the CHD catheter but not in experiments without the CHD catheter. The cholestatic effect of the catheter thus increased the backflux of bile acids from hepatocytes to blood; this effect was not measurable from blood data under normal conditions but was measurable by PET in all experiments. From in vitro studies, it is known that hepatocytes have transporters (MRP3/4 and OSTα/β) for the efflux of bile acids to blood; these can be induced during cholestasis (25,26). Therefore, backflux is an innate ability of hepatocytes. Using 11C-CSar PET, we demonstrated backflux even in the normal state, not just during cholestasis. Because hepatic blood perfusion was constant throughout each PET recording, the Ess/E0 ratio was equal to kBSEP/(kbackflux + kBSEP) (22). This relationship shows that the physiologic significance of backflux is relative to canalicular secretion and that a decrease in kBSEP can cause an apparent increase in kbackflux (22). Using dynamic 11C-CSar PET, we showed that kbackflux in acute cholestasis was, in fact, increased—not only relative to kBSEP but, on average, 20-fold compared with kbackflux in pigs without the CHD catheter. This separation of the individual impacts of kbackflux and kBSEP on Ess cannot be determined from blood measurements alone.
Another interesting finding is that the CHD catheter caused a significant increase in hepatic blood perfusion through a selective increase in portal vein blood flow. Because the hepatic systemic clearance of 11C-CSar is determined by flow, this increase in portal vein blood flow may serve as a regulatory mechanism to secure high clearance of conjugated bile acids despite impaired BSEP—a theory supported by the finding of similar estimates of hepatic systemic clearance from blood data (Clsys) and PET data (Ksys) in both groups of animals. However, the data from the present study do not allow us to draw any conclusions about the potential mechanism behind this finding.
Because there was no accumulation of 11C-CSar in hepatocytes, the highest possible intrahepatic bile volume was estimated as Cliver/Cbile, which yielded a mean maximum volume of 6.0 mL bile/m3 liver tissue (95% CI, 5.8–6.3 mL bile/L liver tissue). This finding, together with a mean of 3.2 mL bile/L liver tissue in stereology studies of human biopsies (27), supports the validity of the mean fractional intrahepatic bile volume of 2.1 mL bile/L liver tissue estimated from the PET data in the present study (Eq. 11).
There was no significant difference between steady-state CHA(t) and steady-state CPV(t) (mean difference, 3%; range, –19% to 31%; P > 0.30), indicating that there was no loss of 11C-CSar in the prehepatic splanchnic bed. This finding is important for future human studies because it is not possible to obtain blood samples from the portal vein in such studies. CPV(t) can then be calculated by use of a model of the transfer of the tracer from that measured in an artery to the portal vein by means of a transfer time constant, β (28,29)—which was, on average, 13 s (95% CI, 6.0–18.6 s) in the present study. Further development of a completely noninvasive method for human studies may be facilitated if arterial blood sampling can be replaced by an image-derived input function, as for 2-18F-fluoro-2-deoxy-d-galactose (30), and if a method without the present need for hepatic vein catheterization can be developed.
11C-CSar was the first conjugated bile acid analog for functional PET studies, but other bile acid tracers, such as N-11C-methyl-tauroursodeoxycholic acid, have since been produced (31). Clinical applications of PET studies with radiolabeled bile acids are likely to include clinical evaluation of patients with cholestatic disorders that affect bile acid uptake, secretion, or bile flow and evaluation of disease progression or treatment efficacy. This technique can also be used to examine how pharmaceuticals may affect hepatobiliary excretory functions.
CONCLUSION
Functional 11C-CSar PET was successful for quantification of the in vivo kinetics of hepatobiliary secretion of conjugated bile acids by external detection in pigs.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. This work was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK074419), the Danish Council for Independent Research (Medical Sciences, 12-125512), and Helga and Peter Korning’s Foundation. No other potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank Ludvik Bass (Department of Mathematics, University of Queensland, Brisbane, Queensland, Australia) for pivotal discussions on data interpretation.
Footnotes
Published online Mar. 10, 2016.
- © 2016 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication December 21, 2015.
- Accepted for publication January 29, 2016.










