Abstract
18F-FDG PET/CT has become the reference standard in oncologic imaging against which the performance of other imaging modalities is measured. The promise of PET/MRI includes multiparametric imaging to further improve diagnosis and phenotyping of cancer. Rather than focusing on these capabilities, many investigators have examined whether 18F-FDG PET combined with mostly anatomic MRI improves cancer staging and restaging. After a description of PET/MRI scanner designs and a discussion of technical and operational issues, we review the available literature to determine whether cancer assessments are improved with PET/MRI. The available data show that PET/MRI is feasible and performs as well as PET/CT in most types of cancer. Diagnostic advantages may be achievable in prostate cancer and in bone metastases, whereas disadvantages exist in lung nodule assessments. We conclude that 18F-FDG PET/MRI and PET/CT provide comparable diagnostic information when MRI is used simply to provide the anatomic framework. Thus, PET/MRI could be used in lieu of PET/CT if this approach becomes economically viable and if reasonable workflows can be established. Future studies should explore the multiparametric potential of MRI.
PET, which was invented by Phelps and Hoffman in the 1970s, was deployed clinically in the late 1980s and early 1990s (1,2). However, clinical acceptance remained limited until integrated PET/CT scanners, developed by Townsend, became commercially available in 2000 (3). The success of PET/CT was swift and spectacular. The number of oncologic PET/CT studies increased from 25,000 in 1996 to more than 2 million in 2014. This success had several reasons. The most important was that the accuracy of 18F-FDG PET/CT for assessing cancer was higher than that of PET or CT alone (4). Another was creation of the landmark National Oncology PET Registry, which resulted in broadening of PET reimbursement by the Centers for Medicare and Medicaid Services (5). In addition, oncologists were better able to visualize and appreciate the molecular information provided by PET when the images were viewed within the anatomic framework provided by CT, and the use of 18F-FDG served as a useful “contrast agent” for radiologists by highlighting anatomically underappreciated yet suggestive lesions. Finally, the cost of PET/CT equipment and imaging was acceptable and only marginally affected cancer care costs, and studies were sufficiently short to maintain high patient throughput (6–8).
The adoption of PET/MRI has been much slower than that of PET/CT. Since its introduction in 2010, approximately 70 systems have been placed worldwide, mostly in academic centers. Equipment pricing, operational costs, and logistics likely account for the slow adoption. In addition, it is difficult to prove a diagnostic advantage when other modalities have already achieved remarkable accuracy. Potential advantages of PET/MRI include high soft-tissue contrast and functional MRI capability. Thus, the promise of PET/MRI includes multiparametric imaging to further improve the diagnosis and phenotyping of cancer. However, rather than focusing on potential synergy between the capabilities of functional MRI and molecular PET, most research has used MRI almost exclusively to provide the anatomic framework for the PET signal. Thus, most studies have compared the diagnostic accuracy of predominantly anatomic PET/MRI with that of 18F-FDG PET/CT in cancer.
The current review serves two main purposes. First, it briefly describes PET/MRI scanner design concepts and discusses technical and operational issues. It then determines whether published data on cancer suggest any significant diagnostic advantages of PET/MRI over PET/CT or vice versa. Such an analysis is justified and informative because close to 50 comparative studies including more than 2,300 patients have now been published.
We used the keywords “PET/CT,” “PET/MRI,” “PET/MR,” and “cancer” to identify studies on PubMed that were published on or before August 20, 2015. Only peer-reviewed prospective or retrospective comparative clinical studies including more than 10 patients were included; we identified a total of 46 comparative clinical studies that included 2,340 cancer patients (Tables 1–7).
Head and Neck Cancer
Lung Cancer and Lung Nodules
Gastrointestinal Cancer and Neuroendocrine Tumors
Gynecologic and Breast Cancer
Prostate Cancer
Lymphoma, Malignant Bone Disease, and Meningioma
Mixed-Cancer Populations
DESIGN OF PET/MRI SYSTEMS
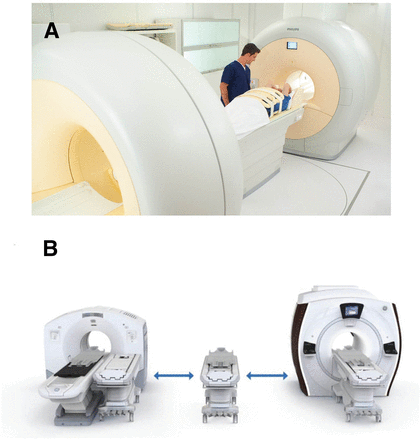
Two fundamentally different PET/MRI designs have been introduced. In the first of these, the PET and MRI data are acquired sequentially, either in a single room or—using a triple-modality PET/CT–MR system—two rooms (Fig. 1). Patient positioning is kept stable by using a shuttle to move the patient from one imaging system to the other. Two advantages are the impossibility of electromagnetic interference between the PET and MRI components and no need for extensive technical modifications of the individual systems. Additional advantages include the ability to use the PET/CT and MRI scanners independently (improving resource use), the ability to acquire MRI data during the uptake period, the ability to save costs by upgrading the MRI and PET technology independently, and preservation of partial functionality if one of the components (PET or MRI) has technical problems. Potential limitations of such systems include image misregistration due to patient motion (including differential bladder filling) and the need for a large installation space. The inability to acquire PET and MRI data simultaneously may also be a drawback, especially if functional processes (MRI) are to be measured and quantified simultaneously with molecular events (PET).
Sequential PET/MRI systems: Ingenuity TF (Philips) (A) and PET/CT+MR trimodality setup (GE Healthcare) (B). Both are connected by scanner bed shuttle system and allow sequential PET and MRI data acquisition at 3-T field strengths. (Courtesy of Philips and GE Healthcare.)
The second PET/MRI design, truly integrated systems, was introduced in 2010 (Fig. 2) (9). In this design, avalanche photodiode–lutetium oxyorthosilicate PET detectors are integrated between the MR body and the gradient coils (9) or, as more recently introduced, semiconductor PET detectors (silicon-based photomultipliers) are used to create time-of-flight capabilities (10,11). We have extensively used the excellent review by Boellaard et al. as source for the following section (11). In simultaneous systems, multiple MR sequences are acquired while PET emission scan data are collected. Thus, imaging time is reduced and image misregistration is minimized (12). MRI-based photon attenuation correction can be derived from segmentation-based or atlas-based algorithms (11). MRI (Dixon, or fast 3-dimensional T1-weighted gradient echo) sequences are obtained to segment tissues into 4 classes (air, lung, fat, and soft tissue) (11,13). “Subsequently, predefined linear attenuation coefficients of PET at 511 keV are assigned to the different tissue classes to obtain an attenuation map for correction of the PET data.” Segmentation-based attenuation correction may lead to truncation and breathing artifacts that can cause errors, and misclassification of bone as soft tissue can occur. These limitations are currently being addressed (11). “Atlas-based attenuation correction has been proposed to retrospectively add bone information to MR-based attenuation correction. However, because this method requires previous knowledge of the atlas data and subsequent correct assignment and registration to the actual patient data, the method fails when patient anatomy deviates from normal (for instance, in patients with large tumors, posttherapy tissue alterations, or anatomic variants)” (11).
Integrated PET/MRI systems: Biograph mMR (Siemens) (A) and Signa PET/MR (GE Healthcare) (B). Both allow for simultaneous PET and MRI data acquisitions at 3-T field strengths. (Courtesy of Siemens and GE Healthcare.)
OPERATIONAL CONSIDERATIONS
As a prerequisite for clinical adoption, PET/MRI protocols need to be efficient. The selection of MR sequences affects workflow and study duration. Various MRI sequences can be obtained while PET data are acquired. However, acquisition of multiple MRI sequences prolongs examination times and limits patient throughput. Imaging protocols to exploit the functional capabilities of MRI are difficult to implement, and the information provided by DWI may be redundant with that provided by 18F-FDG PET (14–16). On the other hand, DWI may add useful information for PET/MRI studies that use highly specific metabolic and receptor-based PET probes to identify changes in tumor cell density in response to treatment. Thus, future research to define the most informative MRI sequences (including DWI) for combination with such targeted PET tracers is needed. Moreover, the impact of functional MRI in conjunction with molecular PET on patient management or outcome remains unknown.
In summary, various PET/MRI configurations that are commercially available come with different advantages and challenges. Clinical imaging protocols should ascertain sufficient patient throughput. The value of functional MRI sequences has not been determined yet.
CLINICAL INDICATIONS FOR PET/MRI
Clinical indications for PET/MRI have not yet been established. The National Comprehensive Cancer Network (NCCN) guidelines list CT and MRI among the first-line imaging modalities for cancer of the head and neck, central nervous system, prostate, and hepatobiliary system (17–20). Thus, if cancer patients are scheduled for MRI studies, it appears reasonable to perform PET/MRI if PET with various metabolic and receptor-based probes can provide relevant information (21–23).
With the exception of studies on prostate cancer and neuroendocrine tumors, most comparative studies have used 18F-FDG and have simply asked whether PET/MRI is feasible and whether the diagnostic accuracy of PET/MRI and PET/CT is comparable. The following sections therefore focus largely on these comparisons in individual types of cancer.
Head and Neck Cancer
The NCCN guidelines support the use of CT, MRI, and PET/CT for staging and restaging of head and neck cancer patients (17). Up to 45% of these present with lymph node involvement at initial diagnosis, and distant metastases are present in 15%. Pulmonary metastases are most common, followed by bone and liver metastases (24). The diagnostic performance of PET/CT has been compared with that of PET/MRI in 7 studies totaling 369 patients (Table 1) (25–31). This relatively large number likely reflects the expectation that because of the exquisite soft-tissue resolution of MRI, PET/MRI may be of particular benefit for assessing head and neck cancer.
T-staging was equivalent between PET/CT and PET/MRI in 3 studies (29–31), but a fourth study found superior lesion discernibility (conspicuity) for PET/MRI (28). Such subjective differences did not result in a change in patient management, yet the improved lesion delineation may be helpful if the infiltration of adjacent structures by primary tumors is of concern (24). In 6 studies, both modalities provided comparable accuracy for detection and characterization of cervical lymph node metastases (25–29,31). Three studies showed no difference (25–27), and the remaining 4 studies provided no information on distant metastasis (28–31).
In 266 patients, comparisons between PET/CT and PET/MRI for recurrent disease showed no significant differences in accuracy (25–31). One report suggested an advantage to PET/MRI in regions of CT artifacts (dental artifacts) (29). Conversely, PET/CT may be beneficial when swallowing or breathing difficulties lead to MR motion artifacts (24). No significant differences in image quality between the modalities were reported (27,28).
In summary, the diagnostic accuracy of PET/CT and PET/MRI was equivalent in 369 patients with head and neck cancer.
Lung Cancer and Pulmonary Lesions
18F-FDG PET/CT is appropriate for pretreatment evaluation of stage I–III non–small cell lung cancer, for additional work-up in stage I–III small cell lung cancer, and for characterization of solid noncalcified or partially nonsolid pulmonary nodules larger than 8 mm (32,33). CT is more accurate than MRI for lung assessments (34,35).
In 6 studies totaling 194 patients with non–small cell lung cancer or lung nodules, PET/MRI and PET/CT performed with comparable accuracy, mainly because of the glucose metabolic information provided by 18F-FDG PET (Table 2) (36–41). Three of the studies (82 patients) compared T-staging (36–38). In one study (10 patients), T-stage was discrepant in 2 patients; however, this did not affect clinical management (36). A larger study (66 patients) reported a high T-stage concordance (38). Lymph node involvement and distant disease were also assessed equally well with the two modalities (36–38). Intermodality agreement was high for M-staging (37). Importantly, none of the subtle differences affected clinical management (36–38).
Fewer 18F-FDG–negative nodules were detected by PET/MRI than by low-dose PET/CT (39). The detection rate of 18F-FDG–negative lung nodules smaller than 10 mm was limited even when fast respiration-gated breath-hold T1-weighted sequences were used—a finding that may be relevant in patients with early pulmonary metastases or patients with head and neck cancer in whom synchronous lung cancer or lung metastases are a concern (40,41). In general, such lesions may denote missed metastatic or primary lung disease, such as lower-grade adenocarcinomas.
In summary, in 194 patients with lung cancer or lung nodules, PET/CT and PET/MRI were of comparable accuracy for TNM staging. PET/CT was superior for lung nodule detection, but PET/MRI was equivalent for characterization of pulmonary lesions in a patient-based analysis.
Gastrointestinal Cancer
Esophageal Cancer
Endoscopic ultrasound, CT, and PET/CT are frequently used in the presurgical work-up to assess resectability, identify and quantify lymph node involvement, and exclude metastatic disease (42,43). Recent guidelines recommend endoscopic ultrasound for T-staging. In contrast, MRI is not listed as a first-line modality and may be reserved for secondary evaluations of the liver or adrenals (43).
In a pilot study of 19 patients, endoscopic ultrasound, CT, PET/MRI and low-dose PET/CT were compared for presurgical staging (Table 3) (44). Endoscopic ultrasound had the highest accuracy for T-staging, whereas PET/MRI was most accurate for N-staging. Unenhanced PET/CT (from a dual-detector CT scanner) was not included in the T-staging analyses. For lymph node staging, accuracy was 83.3%, 75.0%, 66.7%, and 50.0% for PET/MRI, endoscopic ultrasound, PET/CT, and CT, respectively. The difference between PET/MRI and suboptimal, unenhanced 18F-FDG PET/CT was not significant.
Colorectal Cancer
Endorectal ultrasound or abdominopelvic MRI, as well as CT of the chest, abdomen, and pelvis, are recommended for the preoperative staging and restaging of colorectal cancer. PET/CT is considered for evaluating equivocal findings and defining disease extent in patients with suspected or documented potentially resectable metastatic disease. Assessment of treatment responses is another important 18F-FDG PET/CT application (45,46).
The diagnostic accuracy of PET/CT has been compared with that of PET/MRI in 27 patients (Table 3) (47,48). In one study, PET/MRI and low-dose PET/CT were compared for presurgical staging in 2 patients and for restaging in 10 patients (47). Only PET/MRI provided the correct presurgical T-stage in both patients (one with mesorectal fascia involvement). Nevertheless, an analysis of all patients showed comparable sensitivity for PET/CT (71%, 5/7) and PET/MRI (86%, 6/7), with an equivalent specificity (100%, 5/5).
In another study, the N- and M-stages of 180 metastatic colorectal cancer lesions in 15 patients were analyzed (48). In total, 110 of the lesions were malignant, ranging from 0 to 28 lesions per patient (mean, 7). Although PET/MRI included DWI, its diagnostic accuracy was nearly identical to that of 18F-FDG PET/CT (P = 0.28). PET/MRI was, however, superior for assessing 37 hepatic lesions (accuracy, 74% vs. 56%; P < 0.01). Regarding liver lesions undetected by PET/CT, size was not provided, nor was information on whether they were malignant. However, the overall sensitivity, specificity, and accuracy for evaluation of metastatic lesions (N- and M-stages combined) did not differ between the two modalities.
In summary, two studies on colorectal cancer patients (Table 3) reported comparable PET/CT and PET/MRI accuracy for the N- and M-stages in 27 patients. An advantage of PET/MRI for T-staging was reported for two rectal cancer patients. Quite obviously, these numbers are too small for any conclusions to be drawn.
Liver Metastases
The NCCN guidelines list CT as the first-line imaging modality for liver metastases of colorectal cancer (45). MRI is recommended if CT is not adequate. Comparative PET/CT versus PET/MRI data are available from two studies totaling 125 patients (Table 3) (49,50).
In the first, Reiner et al. evaluated 120 (79 malignant and 41 benign) liver lesions in 55 patients using PET/contrast-enhanced CT as the standard of reference (49). Eighty percent of the malignant lesions exhibited increased 18F-FDG uptake; the 18F-FDG–negative lesions were significantly smaller. PET/MRI with T1- and T2-weighted images showed agreement with the standard of reference in 98% of cases. Additional sequences including dynamic contrast-enhanced images or DWI did not change the performance of PET/MRI. Thus, since enhanced CT was used as the reference standard, it appears that PET/MRI and enhanced PET/CT had comparable sensitivity. However, when follow-up was used as the reference, additional metastases were detected in 5 patients on PET/MRI, including DWI and dynamic enhanced MRI, with a corresponding potential impact on management in 10% of the patients. Importantly, this retrospective impact on management analysis did not include false-positive findings, which occurred more frequently with advanced MRI protocols (≤15% of patients). In addition, no state-of-the-art, multiphase enhanced CT protocol was used. It is therefore unknown whether PET/MRI has any benefit over PET/CT in the assessment of liver lesions.
In the second study, Beiderwellen et al. evaluated 97 liver lesions in 70 patients using 18F-FDG PET/MRI and PET/CT (50). All 10 patients with liver metastasis were identified by both modalities. PET/MRI depicted all 71 benign and 26 malignant lesions, whereas 9 benign liver lesions were not identified on PET/CT. Although PET/MRI allowed higher diagnostic confidence (P < 0.001), none of the patients were upstaged or downstaged by PET/MRI.
In summary, in these two studies on 125 patients with liver lesions, PET/MRI and PET/CT detected liver metastases with comparable accuracy. No clear advantage of PET/MRI for detecting and characterizing liver lesions was established.
Gynecologic Cancer
Uterine, ovarian, and cervical cancer is initially diagnosed by ultrasound or biopsy. CT, MRI, and PET/CT are suggested for additional work-up if there is suspected or gross cervical involvement and suspected extrauterine disease (51,52).
Diagnostic accuracy was similar between PET/CT and PET/MRI for detection of primary and recurrent pelvic malignancies in 3 studies that included a total of 69 patients (Table 4) (53–55). In the first study, on 19 patients with recurrent gynecologic cancer, both modalities correctly identified all 58 malignant lesions (57 of which were 18F-FDG–positive), including local and distant sites of recurrence (53). The diagnostic accuracy of PET/MRI and PET/CT on a patient basis was thus identical. The soft endpoint, interpreter confidence, appeared to be higher for PET/MRI than for PET/CT in both malignant (P < 0.01) and benign lesions (P < 0.05) (53).
In the second study, on 26 patients, both modalities accurately identified all primary and recurrent tumors and abdominal metastases (54). Lesion conspicuity was better for PET/MRI (dedicated pelvic sequences) than for enhanced PET/CT.
In the third study, Grueneisen et al. restaged 24 patients with a variety of gynecologic cancers (55). According to the reference standard (histopathology and imaging follow-up), 21 of those 24 patients (88%) had tumor recurrence. Both PET/CT and PET/MRI correctly identified 20 of 21 patients (95%) with tumor relapse.
In summary, in the 69 patients with gynecologic cancer, diagnostic accuracy was comparable between the two modalities (Table 4).
Breast Cancer
MRI is considered for staging in patients with difficult-to-image breasts and those who are inadequately assessed with mammography and ultrasound (e.g., for women with dense breasts, for women with positive axillary nodes and an occult primary tumor presumed to originate in the breast, and for evaluation of the chest well) (56). Diagnostic CT (abdomen and chest) is clinically indicated in patients with suspected metastatic disease. PET/CT is considered an optional additional study when standard imaging results are equivocal or suggestive and is used for breast cancer staging in patients with clinical stage III. It is also used for treatment response assessments (56).
PET/CT was compared with PET/MRI in two studies totaling 85 patients (Table 4) (57,58). In the first, Pace et al. confirmed the feasibility of PET/MRI in 36 breast cancer patients (57). The concordance with PET/CT was high. All 74 18F-FDG–positive lesions were visualized by both modalities. However, imaging findings were not verified by any reference standard.
In the second study, on another 49 patients with 83 lesions, the modalities performed at a comparable level (58). However, T-stage was determined correctly in more patients with PET/MRI (41/50; 82%) than with PET/CT (34/50; 68% [P < 0.05]). No significant differences in N-stage were reported (58).
In summary, PET/MRI is feasible in breast cancer patients (Table 4), but no difference for N- or M-staging was reported. In 7 patients T-stage was better determined with MRI, mainly because of its improved soft-tissue contrast (58).
Prostate Cancer
18F-FDG PET is infrequently used in the workup of patients with prostate cancer. It is therefore not surprising that most published papers describe the use of other PET probes in conjunction with PET/MRI. According to the NCCN guidelines, multiparametric MRI can be used in the staging, characterization, and evaluation of suspected recurrence of prostate cancer (19). CT is listed for clinical assessment after prostate cancer has been diagnosed and is generally considered insufficient to evaluate the prostate gland (19). PET/CT using 11C- or 18F-labeled choline can identify sites of metastatic disease; its sensitivity and specificity for lesion detection in patients with biochemical failure are 85% and 88%, respectively (19,59). The detectability of recurrence sites correlates with serum prostate-specific antigen levels. Several other molecular imaging probes, including 11C-acetate and labeled prostate-specific membrane antigen (PSMA) ligands, are also available for PET imaging (60,61).
The performance of 11C- or 18F-labeled choline and 68Ga-PSMA PET/CT was compared with that of PET/MRI in 3 studies totaling 88 patients (Table 5) (62–64). In the first of these studies, both modalities detected a comparable number of lesions in 36 prostate cancer patients, and image quality was also comparable (64). In the second, 20 patients with advanced prostate cancer were imaged using a 68Ga-labeled PSMA ligand, but the detection rates for PET/CT and PET/MRI were not provided (62). In the third, 11C-choline PET/MRI was compared with PET/CT in 32 patients with prostate cancer (63). Neither lesion number nor lesion conspicuity differed between PET/MRI and PET/CT. T1- and T2-weighted sequences outperformed PET/CT and the Dixon sequence for prostatic lesion allocation and achieved equivalent results for lymph node detection. Solitary lesions were better detected with PET/CT, as may be explained by the fact that PET/CT was performed early whereas PET/MR was usually performed after more than 2 half-lives of 11C had elapsed.
In conclusion, 11C- or 18F-labeled choline and 68Ga-PSMA PET/MRI studies of prostate cancer are feasible. PET/MRI using T1- and T2-weighted sequences was not superior to PET/CT for prostate characterization and bone lesion localization. However, anatomic lesion allocation within the prostate was more accurate with PET/MRI, which may have implications for biopsy planning.
Lymphoma
PET/CT is the imaging modality of choice in all 18F-FDG–avid lymphomas (65–68). CT is the diagnostic modality of choice in lymphomas that are not routinely 18F-FDG–avid. Since 18F-FDG avidity is not predictable in some lymphoma subtypes and since PET/CT includes diagnostic-quality CT, PET/CT imaging can be used for staging and response assessment in all lymphomas.
Heacock et al. investigated 28 (8 Hodgkin and 20 non-Hodgkin) lymphoma patients and identified 51 18F-FDG–avid nodal groups on both modalities (Table 6) (69). DWI alone identified only 32 nodal groups (62.7%). Thus, both PET/CT and PET/MRI (T1-weighted images) were more sensitive than DWI on a lesion-based analysis (P < 0.01). PET/MRI and PET/CT were concordant in all but one patient (agreement of 96.4%).
These early data suggest that PET/MRI and PET/CT can assess disease burden in lymphoma patients with comparable accuracy (Table 3). PET/MRI, even with just basic Dixon and half-Fourier acquisition single-shot turbo spin echo (HASTE) sequences, may be adequate for evaluating treatment response in lymphoma. This may be an important consideration in young patients treated for Hodgkin lymphoma and, in general, in pediatric patients and women of child-bearing age (70,71). However, the cancer risk associated with CT-induced radiation exposure has been controversial (72). This highly publicized topic warrants an in-depth discussion that is beyond the scope of this review. However, the relevance of the linear nonthreshold model that is frequently used to predict cancer risk associated with low-level radiation is highly questionable, and prospective data to substantiate imaging-associated radiation risks in adults are lacking (73).
Neuroendocrine Tumors
Multiphasic CT or MRI can be used for evaluation and surveillance of neuroendocrine tumors of the gastrointestinal tract, lung, and thymus (74). 18F-FDG PET is considered in patients with biopsy-proven neuroendocrine tumors of unknown primary, pheochromocytoma/paraganglioma, and high-grade NET. Somatostatin receptor scintigraphy using a variety of 68Ga-labeled ligands are the standard of care in many parts of the globe for assessing neuroendocrine tumor patients. However, only a few imaging centers provide these diagnostic services in the United States (75).
The diagnostic performance of 68Ga-labeled DOTATOC PET/CT was compared with that of PET/MRI in 2 studies involving 34 patients (Table 3) (76,77). In one cohort, 157 68Ga-DOTATOC–positive lesions were compared (76). MRI detected more liver lesions than CT, an observation of unknown significance since the target, that is, somatostatin receptor–expressing tumors, was detected with identical accuracy by both modalities. Thus, there were no patient- or organ-based differences in lesion detection between PET/CT and PET/MRI (76).
Hope et al. confirmed that MRI identified more liver lesions than CT in 10 patients with neuroendocrine tumors (8 with hepatic involvement) (77). However, the imaging findings were, by study design, not verified by a reference standard, and thus no specificity data were provided. For detection of extrahepatic disease, PET/MRI was equivalent to PET/CT (77).
Overall, both modalities performed equally well with regard to image quality, and sensitivity was comparable between the two modalities in 34 NET patients. It remains unknown whether PET/MRI with probes for neuroendocrine tumors detects more lesions than PET/CT.
Metastatic Bone Disease
Skeletal scintigraphy followed by plain radiography, if necessary, is suggested for staging of patients at high risk for bone metastasis. CT and MRI are listed as additional modalities for indeterminate radiographic findings. 18F-FDG PET/CT is considered a modality complementary to bone scintigraphy. MRI may be more sensitive for detecting early lesions and marrow-based metastases than plain radiography, CT, or radionuclide bone scanning (78).
18F-FDG PET/CT was compared with PET/MRI in 3 studies totaling 295 patients with suspected bone metastases (Table 6) (79–81).
In the first, on a mixed population of 119 patients with head and neck cancer, breast cancer, gastrointestinal cancer, sarcomas, and others, 98 bone lesions were identified both on PET/CT and on T1-weighted turbo spin echo PET/MRI. Lesion identification was nearly identical for PET/CT and PET/MRI. In ratings of anatomic delineation, T1-weighted turbo spin echo imaging performed significantly better than CT (P = 0.0001) or T1-weighted Dixon in-phase MRI (P = 0.0002). Nevertheless, no significant differences in correct classification of malignant bone lesions between the two modalities were reported (79).
In the second study, PET/CT identified 45 of 48 bone metastases (94%) whereas PET/MRI detected all bone metastases (80). PET/MRI showed osseous metastases in one more patient. In that patient, with non–small cell lung cancer, a bone metastasis showing intensely increased 18F-FDG uptake (a lesion with an SUVmax of 4.5 was described as “moderate” in the paper) was rated as “indeterminate” on PET/CT, whereas on PET/MRI the bone metastasis was identified because of diffusion restriction, hyperintensity on T2-weighted images, and increased tracer uptake in comparison to the surrounding tissue. Other than improved lesion conspicuity, no significant differences in lesion detection were reported (80).
In the third study, by Catalano et al. (81), improved detection of osseous metastases was reported for PET/MRI. Bone involvement was detected in more patients, and more individual bone lesions were identified. The multiparametric MRI protocol was elaborate and required average examination times of more than 90 min and up to 2 h in many patients. It included whole-body enhanced axial and coronal T1-weighted sequences as well as several DWI sequences (axial T2-weighted HASTE, axial PET, axial fused HASTE-PET, axial b-800 DWI, apparent diffusion coefficient map, coronal short-T1 inversion recovery, and coronal T1 in-phase and out-of-phase Dixon).
In summary, the currently available data are still limited. Using simple T1-weighted turbo spin echo images, Eiber et al. found no differences in bone lesion detectability (79). However, there may be a diagnostic advantage to PET/MRI in detecting bone metastases if multiparametric MRI protocols are used. To arrive at reasonable and feasible study durations, it would be important to identify and prospectively test those sequences that proved most beneficial in the retrospective study by Catalano et al. (81).
Pediatric Cancer
Because of concerns about radiation exposure, pediatric oncology may become a key application for PET/MRI (82). However, comparative data have been reported for only 18 pediatric patients (Table 7) (83). Lesion detection rates were nearly identical. One lung lesion with focal 18F-FDG uptake seen on PET/CT was missed on PET/MRI because of incorrect attenuation correction. MRI provided additional value in characterizing soft-tissue lesions and in detecting malignant bone marrow infiltration not visible on PET (total number not provided). These differences led to a true upstaging in 2 patients and detection of cancer recurrence in 2 other patients, with an obvious potential impact on patient management. However, in 2 patients with sarcoma, CT showed multiple lung metastases that were only partly visible on MRI. A clear advantage of one modality over the other (with the exception of the reduced radiation exposure associated with PET/MRI) has therefore not yet been established.
Central Nervous System Tumors
Software fusion of 18F-FDG PET and MRI studies of the brain has been performed successfully for many years. Integrated PET/MRI would be a natural extension of this application, especially with non–18F-FDG tumor tracers and for evaluating progressive neurodegenerative disease using amyloid and tau radiotracers. However, comparisons have thus far been limited to patients with meningioma.
MRI is the gold standard for imaging central nervous system cancer, whereas CT should be used in patients with contraindications to MRI (18). In one study of 15 patients, all meningiomas were detected with 68Ga-DOTATOC PET/CT and PET/MRI (Table 6) (84). Studies on patients with glioblastoma have not yet been published.
Mixed-Cancer Populations
Thirteen studies that included various cancer types have been published (996 patients with cancer of the colon, breast, pancreas, esophagus, gynecologic system, or head and neck or with melanoma, lymphoma, leukemia, or other types of cancer) (Table 7) (10,83,85–95). Various PET probes, including 18F-FDG, 68Ga-DOTATATE, 6-18F-fluoro-l-dopa, and 18F-choline, were used. Whole-body PET/MRI was feasible and lesion detection comparable in most reports.
One of these studies compared TNM staging and showed that 18F-FDG PET/CT and PET/MRI were of equivalent diagnostic accuracy in 73 patients with solid tumors (Table 7) (94). The impact of 18F-FDG PET/MRI on patient management was assessed retrospectively by Catalano et al. (Table 6) (88), who suggested a significant additional impact of 18F-FDG PET/MRI on patient management.
SUMMARY AND FUTURE PERSPECTIVES
The current data document that PET/MRI protocols are feasible across all types of cancer. Clear diagnostic advantages of PET/MRI, when used mainly for providing the anatomic framework, have not been established and will be difficult to demonstrate given the high accuracy of PET/CT (96). Multiparametric but not standard PET/MRI may have advantages for better allocation of bone metastases and for localizing intraprostatic sites of disease involvement. Conversely, the superiority of PET/CT for lung assessment is relevant across many types of cancer. It seems reasonable to use PET/MRI for those types of cancer that are routinely imaged with MRI when the addition of PET (with various probes) can provide added value.
PET/MRI is an expensive technology that should not be used simply to replace PET/CT. Clearly, the multimodal capabilities of functional MRI should be exploited and tested for their added value in better understanding and characterizing cancer. Processes such as tumor perfusion at baseline and in response to therapy can be studied with MRI, which may provide extremely useful insights into drug delivery and thus effectiveness. Target expression and inhibition may be determined by combining PET and MRI. Thus, the efficient and selective incorporation of advanced MRI sequences and dedicated organ-specific scanning in PET/MRI should be explored to fully realize the diagnostic potential of this new modality. PET/CT can be further improved by using advanced CT protocols with oral and intravenous (multiphase) contrast protocols. Future comparative studies between PET/CT and PET/MRI therefore need to use such advanced protocols to permit appropriate comparisons between the two modalities.
Footnotes
Published online Jan. 7, 2016.
Learning Objectives: On successful completion of this activity, participants should be able to (1) describe the different PET/MRI designs available for cancer assessment; (2) understand how photon attenuation correction for PET/MRI is done; and (3) evaluate whether PET/MRI provides diagnostic advantages over PET/CT in cancer.
Financial Disclosure: Dr. Czernin has ownership (unrelated to the paper) in Sofie Biosciences, Momentum Biosciences, and Trethera Corporation. Dr. Herrmann has ownership (unrelated to the paper) in SurgicEye and Sofie Biosciences and is a member of the advisory board of OctreoPharm Sciences GmbH. The authors of this article have indicated no other relevant relationships that could be perceived as a real or apparent conflict of interest.
CME Credit: SNMMI is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to sponsor continuing education for physicians. SNMMI designates each JNM continuing education article for a maximum of 2.0 AMA PRA Category 1 Credits. Physicians should claim only credit commensurate with the extent of their participation in the activity. For CE credit, SAM, and other credit types, participants can access this activity through the SNMMI website (http://www.snmmilearningcenter.org) through March 2019.
- © 2016 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.
- 23.↵
- 24.↵
- 25.↵
- 26.
- 27.↵
- 28.↵
- 29.↵
- 30.
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.
- 67.
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.
- 87.
- 88.↵
- 89.
- 90.
- 91.
- 92.
- 93.
- 94.↵
- 95.↵
- 96.↵
- Received for publication September 29, 2015.
- Accepted for publication January 5, 2016.