Abstract
Transforming growth factor–β (TGF-β) signaling is involved in glioma development. The monoclonal antibody fresolimumab (GC1008) can neutralize all mammalian isoforms of TGF-β, and tumor uptake can be visualized and quantified with 89Zr-fresolimumab PET in mice. The aim of this study was to investigate the fresolimumab uptake in recurrent high-grade gliomas using 89Zr-fresolimumab PET and to assess treatment outcome in patients with recurrent high-grade glioma treated with fresolimumab. Methods: Patients with recurrent glioma were eligible. After intravenous administration of 37 MBq (5 mg) of 89Zr-fresolimumab, PET scans were acquired on day 2 or day 4 after tracer injection. Thereafter, patients were treated with 5 mg of fresolimumab per kilogram intravenously every 3 wk. 89Zr-fresolimumab tumor uptake was quantified as maximum standardized uptake value (SUVmax). MR imaging for response evaluation was performed after 3 infusions or as clinically indicated. Results: Twelve patients with recurrent high-grade glioma were included: 10 glioblastomas, 1 anaplastic oligodendroglioma, and 1 anaplastic astrocytoma. All patients underwent 89Zr-fresolimumab PET 4 d after injection. In 4 patients, an additional PET scan was obtained on day 2 after injection. SUVmax on day 4 in tumor lesions was 4.6 (range, 1.5–13.9) versus a median SUVmean of 0.3 (range, 0.2–0.5) in normal brain tissue. All patients showed clinical or radiologic progression after 1–3 infusions of fresolimumab. Median progression-free survival was 61 d (range, 25–80 d), and median overall survival was 106 d (range, 37–417 d). Conclusion: 89Zr-fresolimumab penetrated recurrent high-grade gliomas very well but did not result in clinical benefit.
High-grade gliomas are rapidly progressive brain tumors that are divided into anaplastic gliomas and glioblastomas multiforme (GMB) on the basis of their histopathologic features. The 5-y survival rates for anaplastic oligodendroglioma, anaplastic astrocytoma, and GBM are 49%, 25%, and 5%, respectively (1). In addition to surgery, the standard treatment of gliomas is currently based on tumor cell death induction by radiotherapy and chemotherapy. As a result of the modest treatment results, novel strategies for the treatment of malignant glioma are needed.
Transforming growth factor–β (TGF-β) acts as a tumor promoter in advanced tumors, where it induces proliferation and metastasis and suppresses the immune response (2). TGF-β and its receptors are overexpressed in GBM, and TGF-β signaling is involved in multiple steps of GBM development and invasion (3–5). Plasma TGF-β levels are elevated in GBM patients and decrease after surgical tumor resection (6). In addition, progression-free survival (PFS) and overall survival are decreased in glioma patients with high levels of phosphorylated SMAD2 (p-SMAD2), the substrate of TGF-β receptor I, compared with glioma patients with low levels of p-SMAD2 (7). These features make TGF-β a promising target molecule for therapeutic approaches in recurrent glioma; therefore, several TGF-β-inhibitors are under investigation in this setting (8).
Fresolimumab (GC1008) is a monoclonal antibody capable of neutralizing all mammalian isoforms of TGF-β (i.e., 1, 2, and 3) (9). In a phase 1 study with fresolimumab in patients with melanoma and renal cell carcinoma, 6 patients achieved stable disease and one patient had a partial response (10). In a phase 2 study in 13 mesothelioma patients, stable disease was seen in 3 patients at 3 mo (11).
Current standard-of-care and experimental treatment results in patients with recurrent high-grade glioma are disappointing. It is often suggested that this is because of the impermeability of the blood–brain barrier, which may prevent drugs from reaching the tumor (12). For therapeutic success in brain tumors, it is essential for a monoclonal antibody such as fresolimumab to reach the target site in the brain. In tumor xenograft models, tumor uptake could be visualized and quantified with 89Zr-fresolimumab PET (13). Therefore, the aim of this study was to visualize and quantify fresolimumab uptake in recurrent high-grade glioma using 89Zr-fresolimumab PET. In addition, we evaluated the effect of treatment with fresolimumab in patients with recurrent high-grade glioma.
MATERIALS AND METHODS
Patients
Adult patients who had recurrent glioma with one or more contrast-enhancing lesions of at least 20 mm on MR imaging were eligible. The main additional inclusion criteria were a World Health Organization performance score of 0–2; adequate bone marrow; coagulation; kidney and liver function; and negative tests for hepatitis B, C, and HIV. Previous surgery, radiotherapy, chemotherapy, or investigational agents should have been more than 4 wk before inclusion (>6 wk for nitrosourea or monoclonal antibodies), and patients must have recovered from previous treatment. Main exclusion criteria were a history of ascites or pleural effusions, active hypercoagulability states or use of anticoagulants, hypercalcemia, pregnancy or nursing, diagnosis with other malignancies (unless curatively treated), organ transplants, immunosuppressive therapy, active infection, autoimmune disease, and other significant uncontrolled medical illnesses.
This study has been approved by the local medical ethical committee and registered in a clinical trial register (trial registration ID, NCT01472731). All patients gave written informed consent. A data safety monitoring board reviewed progress and safety during the study.
Treatment
Patients were treated with a 5 mg/kg dose of fresolimumab (Genzyme; Sanofi-Aventis Oncology) intravenously every 3 wk until the occurrence of radiologic or clinical progression or unacceptable toxicity. Fresolimumab was administered over 90 min for the first infusion, then over 60 min, and finally over 30 min if no infusion-related reactions occurred. Within 30 min before infusion, patients received acetaminophen (500 mg) and clemastine (2 mg) as premedication. All adverse events were recorded and graded according to the Common Terminology Criteria for Adverse Events, version 4. PFS and overall survival were calculated from date of informed consent to date of disease progression on MR imaging, clinical progression, or death.
Imaging
Conjugation and radiolabeling of fresolimumab were performed under good manufacturing conditions as previously described (13). Before the start of treatment with fresolimumab, patients were injected with 37 MBq (5 mg) of 89Zr-fresolimumab. The radioactive dose of 37 MBq and the protein dose of 5 mg result in a specific activity of 7.4 MBq/mg. Thereafter, patients were observed for 2 h for possible infusion-related reactions.
89Zr-fresolimumab PET scans were acquired on day 4 after injection. To assess the tumor accumulation of 89Zr-fresolimumab over time, an additional scan was acquired on day 2 after injection in some patients. Normal organ distribution of 89Zr-fresolimumab was assessed using whole-body PET scans. The images were acquired using 2 PET camera systems (ECAT HR+ [Siemens Medical Systems] and mCT Biograph [Siemens Medical Systems]). Acquisition time for the ECAT HR+ PET camera was 10 min per bed position on day 2 after injection (of which 20% is transmission time). On day 4 after injection, imaging time was prolonged to 12 min per bed position to correct for decay time. For the mCT camera, imaging time was shorter (5 min per bed position). All scans were reviewed and analyzed by a nuclear medicine physician and an investigator. All attenuation-corrected PET images and MR imaging series (gadolinium-enhanced T1-weighted imaging, performed within 4 wk before the start of the study) were retrospectively fused using a commercially available software program (esoft, 3D fusion; Siemens Medical Solutions) on a Siemens workstation (syngo MMWP; Siemens Medical Solutions) to identify tumor lesions. The 2 datasets were aligned on the basis of mutual information using the anatomic contours of the loaded datasets. Regions of interest were drawn around the tumor lesions on the PET scans. In normal organs, regions of interest were drawn in the same area of the organs for all patients. 89Zr-fresolimumab uptake was quantified using AMIDE Medical Image Data Examiner software, version 0.9.2 (Stanford University), to calculate the standardized uptake value (SUV) (14). The SUVmax of the tumor lesions and the SUVmean of normal organs, including blood (measured in the sinus confluens and the iliac artery), were calculated.
Follow-up brain MR imaging (1.5 T using T1-, T2-, and contrast-enhanced 3D T1-weighted gradient-echo sequences) was performed after every 3 treatment cycles (every 9 wk) or as clinically indicated. MR imaging data for this study were assessed by a neuroradiologist using the criteria of Macdonald et al. for tumor response evaluation (15).
Plasma Pharmacokinetics and Biomarkers
Heparin plasma samples for 89Zr-fresolimumab pharmacokinetics were collected from patients 1 h after injection and at the time of PET scanning. Plasma samples were counted in a γ counter, and the tracer concentration in plasma was calculated using a calibration graph.
Before the start of fresolimumab treatment, citrate plasma samples were collected. Blood samples were drawn without a tourniquet when possible, immediately placed on ice, and centrifuged at 2,500g for 30 min at 4°C with the brake turned off. Plasma samples were stored at −70°C. In these samples, total TGF-β1 was analyzed using a human TGF-β1 immunoassay (Quantikine; R&D Systems).
An analysis of p-SMAD2 as a readout of TGF-β signaling was performed in archival paraffin-embedded primary tumor tissue for all patients. Formalin-fixed, paraffin-embedded 3-μm tissue sections were mounted on microscope slides and dried overnight at 55°C. Tissue sections were dewaxed in xylene and rehydrated in a graded series of ethanol. Sections were subjected to microwave pretreatment with pH 6.0 citrate buffer for staining of p-SMAD2 (catalog no. 3101; Cell Signaling Technology, Inc.). Subsequently, sections were treated with 0.3% H2O2 for 30 min, blocked for 1 h with 2% bovine serum albumin to reduce nonspecific antibody binding, and incubated with primary antibody. All antibody solutions were made in phosphate-buffered saline with 1% bovine serum albumin and 0.1% TritonX-100 (The Dow Chemical Co.). Incubation at 4°C overnight was followed by incubation with goat antirabbit antibody conjugated to peroxidase (Dako) and subsequently with rabbit antigoat antibody conjugated to peroxidase (Dako). Staining was visualized by 3,3′-diaminobenzidine, and sections were counterstained with hematoxylin and mounted. As a negative control, primary antibody was omitted and incubations were performed as described previously.
Statistical Analysis
In the protocol, 2 stopping rules were defined. The study would be terminated after the inclusion of 6 patients if no 89Zr-fresolimumab uptake was seen on the PET scan, and after the inclusion of 12 patients if treatment with fresolimumab showed no clinical benefit. If a clinical benefit was seen, a maximum of 20 patients could be included. Statistical analyses were performed using the Pearson correlation test and the Mann–Whitney U test using SPSS statistics 20 (IBM). Data are presented as median with range unless stated otherwise. Two-sided P values of 0.05 or less were considered to indicate significance. Graphs were made using Prism, version 5.00 (GraphPad), for Windows (Microsoft).
RESULTS
Patients and Treatment
Twelve patients with recurrent high-grade glioma (9 primary GBM, 1 secondary GBM [World Health Organization grade IV], 1 secondary anaplastic oligodendroglioma, and 1 secondary anaplastic astrocytoma [grade III]) were enrolled in this study (Table 1). Patients were previously treated with 2 lines of treatment (1–8).
Patient Characteristics
Two patients received 1 infusion of fresolimumab, 5 patients received 2 infusions, and 5 patients received 3 infusions. All patients showed clinical progressive disease during treatment or progressive disease on the first MR imaging scan obtained during treatment. PFS was 61 d (range, 25–80 d), and overall survival was 106 d (range, 37–417 d). In the absence of clinical benefit, the study was closed after the first 12 patients.
No adverse events were related to tracer injection. In 12 patients, 69 nonhematologic adverse events, mostly grade 1 or 2 and mostly related to progression of the disease, were observed during the study. Thirteen hematologic grade 1 adverse events were registered. The most common adverse events were neurologic deterioration, headache, skin disorders, nausea, and fatigue (Table 2). Adverse events that were considered as possibly related to fresolimumab were acneiform rash (grade 1, 1 patient), dry skin (grade 1, 1 patient), fatigue (grade 2, 2 patients), thrombocytopenia (grade 1, 1 patient), and epistaxis (grade 1, 1 patient). Four serious adverse events were recorded, of which 3 were neurologic worsening related to progressive disease and 1 was pain related to an osteoporotic vertebra fracture, all of which were assessed as unrelated to fresolimumab.
Hematologic Adverse Events and Most Common Nonhematologic Adverse Events
In 4 patients, no MR imaging after treatment was performed because of clinical deterioration. In 2 patients, suspected dispersed hemorrhagic spots were seen in the tumor on MR imaging after treatment. A relationship with fresolimumab could not be excluded, although one of these patients also had a second course of radiotherapy before entrance into the study.
Imaging
All 12 patients underwent at least a brain-only PET scan on day 4 after injection. Seven patients underwent a whole-body scan. Four patients underwent a whole-body scan on both day 2 and day 4 after injection. The mean interval between date of consent and injection of the PET tracer was 7 d (range, 0–15 d).
In all patients, uptake of 89Zr-fresolimumab was seen in tumor lesions (n = 16). The mean SUVmax in tumor lesions on day 4 was 4.6 (range, 1.5–13.9), which was higher than the SUVmean of normal brain tissue (0.3 [range, 0.2–0.5]) (P < 0.01). The SUVmean was 3.0 (range, 2.0–6.2) in the blood of the sinus confluens. In patients who had a whole-body scan, the SUVmean of normal organs was the highest in the heart (8.3 [range, 6.4–8.9]), followed by the liver (7.1 [range, 5.4–11.2]) and the kidneys (5.5 [range, 3.4–6.6]) (Fig. 1). In 8 patients, not all tumor lesions showed 89Zr-fresolimumab uptake. Most tumor lesions that did not show uptake were small (<10 mm on MR imaging). In 3 patients, no uptake was seen in larger gadolinium-enhanced lesions of 13, 18, and 12 mm. The latter 2 lesions were found in previously irradiated areas, and one of these was not visible on the follow-up MR imaging (Fig. 2). In all 4 patients who underwent a PET scan on both day 2 and day 4 after injection, the tumor-to-blood ratio (measured in the sinus confluens) increased from day 2 to day 4 after injection (Fig. 2). There was no correlation between tumor uptake and PFS or overall survival.
(A) Representative example of 89Zr-fresolimumab PET on day 4 and uptake in brain tumor (arrow). (B) Uptake of 89Zr-fresolimumab in different organs (SUVmean) and tumor (SUVmax) on day 4 after tracer injection. Blood pool uptake was measured in sinus confluens. Blood, brain, and tumor values were measured in 12 patients, other organs in 7 patients.
(A) PET/MR images of patient with 2 contrast-enhancing lesions. High uptake is visible in frontal lesion (left) but not in previously irradiated occipital lesion (right). (B) PET/MR images of patient with 2 contrast-enhancing lesions. SUVmax was 5.5 in progressive right frontal lesion and 2.1 in previously irradiated right paraventricular lesion. (C) Whole-body PET scans on days 2 (left) and 4 (right) showing increase in SUVmax from 4.0 to 5.5 in frontal brain lesion (arrows). Tumor-to-blood ratio increased from 0.8 to 1.2. (D) Tumor-to-blood ratios on 89Zr-fresolimumab PET in 4 patients on days 2 and 4.
Plasma Pharmacokinetics and Biomarkers
The mean plasma concentrations of 89Zr-fresolimumab at 1 h, 2 d, and 4 d after injection were 1.87 μg/mL (range, 1.20–2.30 μg/mL), 1.31 μg/mL (range, 0.96–1.76 μg/mL), and 1.06 μg/mL (range, 0.72–1.38 μg/mL), respectively. When corrected for the injected dose, the extrapolated maximum concentration per dose was 0.37 μg/mL/mg (range, 0.23–0.41 μg/mL/mg) (n = 10).
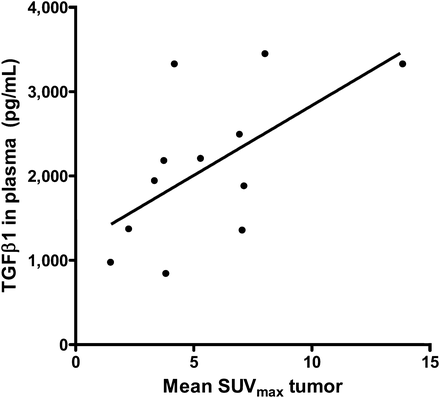
Pretreatment plasma TGF-β1 levels were 2,058 pg/mL (range, 837–3,444 pg/mL) and correlated with mean SUVmax in the tumor lesions 4 d after injection (r = 0.61, P = 0.04) (Fig. 3). Staining in primary tumor tissue with p-SMAD2 was positive for all tumors but also for normal brain tissue (Fig. 4).
Correlation between TGF-β1 in plasma and mean SUVmax of 89Zr-fresilimumab in brain tumor lesions on day 4 after injection (r = 0.61, P = 0.04).
GBM with central necrosis on hematoxylin and eosin (A) and p-SMAD2 (B) staining of same area.
DISCUSSION
To our knowledge, this was the first study to show tumor uptake of a radiolabeled antibody in patients with recurrent high-grade gliomas, indicating that fresolimumab does reach its target destination in the brain. Unfortunately, monotherapy with fresolimumab did not result in an antitumor effect.
The median SUVmax of 4.6 found in the gliomas is comparable to the SUVmax of 5.8 (range, 1.7–15.1) found with 89Zr-bevacizumab PET in metastatic lesions in patients with neuroendocrine tumors (16). The maximum concentration per dose of 89Zr-fresolimumab 1 h after injection of 0.37 μg/mL/mg is comparable to the pharmacokinetic results of an earlier study with fresolimumab (17). This indicates that the radiolabeled antibody has a maximum concentration similar to that found for fresolimumab in other studies. Three contrast-enhancing lesions larger than 10 mm did not take up 89Zr-fresolimumab. Two were found in previously irradiated areas, and one of these disappeared on follow-up MR imaging. These lesions are suspected to represent radionecrosis instead of viable tumor tissue, which might be the reason for the lack of TGF-β and uptake of 89Zr-fresolimumab. In all patients who underwent a whole-body PET scan on both day 2 and day 4 after injection, the tumor-to-blood ratio increased. This increase in ratio supports the notion of tumor-specific uptake. This pattern of tumor accumulation and increasing tumor-to-blood ratios over time was also seen in our preclinical study with 89Zr-fresolimumab and in brain metastases in a clinical study with 89Zr-trastuzumab in patients with metastatic breast cancer (13,18). Taken together, these findings suggest that 89Zr-fresolimumab uptake not only was a reflection of antibody leakage due to a damaged blood–brain barrier but was tumor-specific and TGF-β–driven.
In earlier studies, the uptake of gemcitabine and GRN1005 in patients with recurrent glioma was shown by analyzing tumor tissue obtained during surgery (19,20). However, performing tumor biopsies is often not feasible in this patient group, and tumor characteristics may change over time. PET scanning can be a noninvasive alternative for exploring potential targets that may be influenced by drugs and showing tumor penetration of drugs.
Treatment with fresolimumab was generally well tolerated, without infusion-related reactions. Most adverse events were grade 1 or grade 2 and related to progression of the disease. Unfortunately, no clinical benefit was observed in this small and often extensively pretreated patient group in which only one dose of fresolimumab was tested. Possible effects of this treatment in higher doses can therefore not be excluded. The median PFS was 61 d, which is comparable to the PFS of the physician-choice chemotherapy arm in recurrent GBM in a recently conducted randomized phase 3 trial (21).
In all archival tumor samples, p-SMAD2 was positive, indicating that the TGF-β pathway was active in the tumors. In gliomas, multiple signaling pathways are activated, and inhibition of just one pathway might be insufficient for a response (22). Recently, other clinical studies using TGF-β inhibition in glioma patients have been published. Traberdersen is an antisense oligodeoxynucleotide that inhibits TGF-β2. In a randomized grade 2B study, traberdersen was administered intratumorally by convection-enhanced delivery and compared with standard chemotherapy in patients with recurrent or refractory high-grade glioma. Six-month tumor control rates did not significantly differ among the entire study population (anaplastic astrocytoma and GBM). A prespecified anaplastic astrocytoma subgroup analysis showed a significant benefit regarding the 14-mo tumor control rate for traberdersen versus chemotherapy (23). A phase 1 study with LY2157299 (a TGF-β1 receptor kinase inhibitor) showed confirmed responses in treatment-refractory gliomas in 3 of 28 patients (24). Therefore, TGF-β remains a potential interesting target in glioma patients, and more combination studies are welcomed.
CONCLUSION
This study proved that an antibody against TGF-β reaches recurrent high-grade gliomas. Although no treatment benefit was seen, this finding could be exploited for further development of recurrent high-grade glioma treatment with antibodies or antibody–drug conjugates.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. This work was supported by Genzyme (Sanofi-Aventis Oncology), including a grant for PET scans that was made available to the UMCG. Joseph Pearlberg was employed by Sanofi Aventis Oncology, Cambridge, MA. He is currently working at Infinity Pharmaceuticals, Cambridge, MA. No other potential conflict of interest relevant to this article was reported.
Acknowledgments
This work was presented as a poster at the ASCO Annual Meeting, 2013.
Footnotes
Published online Jul. 1, 2015.
- © 2015 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication January 27, 2015.
- Accepted for publication June 16, 2015.