Abstract
Successful management of solid tumors in children requires imaging tests for accurate disease detection, characterization, and treatment monitoring. Technologic developments aim toward the creation of integrated imaging approaches that provide a comprehensive diagnosis with a single visit. These integrated diagnostic tests not only are convenient for young patients but also save direct and indirect health-care costs by streamlining procedures, minimizing hospitalizations, and minimizing lost school or work time for children and their parents. 18F-FDG PET/CT is a highly sensitive and specific imaging modality for whole-body evaluation of pediatric malignancies. However, recent concerns about ionizing radiation exposure have led to a search for alternative imaging methods, such as whole-body MR imaging and PET/MR. As we develop new approaches for tumor staging, it is important to understand current benchmarks. This review article will synthesize the current literature on 18F-FDG PET/CT for tumor staging in children, summarizing questions that have been solved and providing an outlook on unsolved avenues.
- pediatric oncology
- positron emission tomography
- PET/CT
- pediatric cancer imaging
- cancer staging
- pediatric lymphoma
- pediatric sarcoma
The use of 18F-FDG PET and PET/CT imaging as a clinical tool for staging and restaging some but not all pediatric tumors has been established. Several studies have demonstrated improved sensitivities and specificities for 18F-FDG PET/CT compared with all collective standard staging procedures, specifically for patients with lymphomas, sarcomas, and head and neck cancers (1–4). However, exposure of children to radiation through 18F-FDG PET/CT is of concern to the pediatric imaging community (5,6), and various low-dose approaches are being pursued (7–9). A thorough understanding of the state of knowledge is needed to preserve the advantages of current 18F-FDG PET/CT staging tests and create new imaging tests without compromises.
To this end, we performed a comprehensive MEDLINE literature search using the terms (child OR teen OR adolesc OR pediatric OR infant OR newborn OR neonat) AND (“Positron-Emission Tomography and Computed Tomography” OR “pet/ct” OR “Hybrid Pet and CT” OR “integrated pet ct” OR “SPECT and CT” OR “pet ct” OR “CT and PET”). This search identified 762 articles, which were further filtered regarding the following inclusion criteria: original research articles related to 18F-FDG PET or 18F-FDG PET/CT, pediatric or adolescent population only (up to age 23 y), malignant diseases, and minimum sample size of 10 cases per article. This led to 65 original research articles, which will be reviewed and summarized in this article, including 11 articles that were further evaluated with a metaanalysis. The metaanalysis provided information about the sensitivity, specificity, and accuracy of 18F-FDG PET or 18F-FDG PET/CT for staging and therapy response assessment of malignant lymphomas, with histopathology or clinical and imaging follow-up as the reference standard.
Although staging tests for pediatric cancers were originally performed with stand-alone 18F-FDG PET scanners (10–12), these have been largely replaced by integrated 18F-FDG PET/CT scanners (13,14). The added CT component improves the diagnostic accuracy of PET alone by adding a higher anatomic resolution to the acquired image information, thus improving lesion detection and characterization (15). Overall, the reported sensitivities of 18F-FDG PET/CT for tumor staging in children are 90%–97% and the reported specificities are 99%–100% (16–18). These data refer to specific subsets of common pediatric malignancies, mainly lymphomas, sarcomas, and small cell neoplasms, and cannot be generalized to other pediatric malignancies. When the findings of different imaging tests were discrepant, 18F-FDG PET/CT was found to be the accurate modality in 90% of evaluated cases (16). Importantly, the results of PET/CT scans demonstrated an impact on clinical decisions and patient management. For example, in a study on pediatric patients with lymphoma, Ewing sarcoma, primitive neuroectodermal tumor, or medulloblastoma, 18F-FDG PET/CT changed the staging results in 61% of cases when compared with conventional imaging modalities (including CT, MR imaging, and ultrasonography) (19). Diagnostic information obtained from 18F-FDG PET scans changed management in 24% of pediatric oncology patients, including those with lymphoma, sarcoma, central nervous system tumor, and plexiform neurofibroma (20). In this article, we will review how 18F-FDG PET and PET/CT contributed to the staging and treatment monitoring of malignant tumors in pediatric patients.
MALIGNANT LYMPHOMA
Lymphoma is the third most common malignancy in the pediatric population (after leukemia and malignant brain tumors), comprising nearly 15% of childhood malignancies and 1,700 new diagnoses per year (53% Hodgkin lymphoma [HL] and 47% non-Hodgkin lymphoma [NHL]) (21). Classic HL accounts for more than 85% of cases of HL, whereas nodular lymphocyte-predominant HL is a less common subtype. NHL is a more heterogeneous group and includes high-grade lymphomas such as Burkitt lymphoma and Burkittlike tumors, which are more common in younger patients (5–14 y); diffuse large B-cell lymphomas, which are more common in older children and adolescents (15–19 y); and lymphoblastic lymphoma and anaplastic large cell lymphoma (1.4–13 y) (22). Rarer subtypes are also seen. Indolent lymphomas are uncommon in children (<5% of pediatric NHL), unlike adults (23). The 5-y survival rate is 95% for HL and 78% for NHL (21,24).
Initial Staging
Many studies have shown that 18F-FDG PET or PET/CT is superior for staging malignant lymphomas when compared with conventional imaging modalities, including contrast-enhanced CT, MR imaging, bone scintigraphy, ultrasonography, and 67Ga-scintigraphy (11,12,14,25–27). Most histologic subtypes of NHL in children are of high histologic grade and show strong 18F-FDG uptake (28,29). Indolent lymphomas, such as extranodal marginal zone lymphoma, small lymphocytic lymphoma, and peripheral T-cell lymphoma, are rare in children. These subtypes generally show little or no 18F-FDG uptake (28,30) or remain localized for long periods (31) and, thus, are usually not referred for 18F-FDG PET/CT staging examinations (32).
The sensitivities and specificities of 18F-FDG PET/CT or 18F-FDG PET for initial staging of malignant lymphomas are 96%–99% and 95%–100%, respectively (Table 1) (11,12,14,33,34). 18F-FDG PET is more sensitive than CT in detecting nodal and extranodal lesions in HL and NHL, including lesions in the spleen and bone marrow (Fig. 1) (11,13). CT, on the other hand, is more sensitive in the evaluation of pulmonary involvement: the positron range of 18F-FDG limits the spatial resolution of PET, and continuous breathing during PET data acquisition impairs the detection of small pulmonary nodules (11,13). Although lung metastases are not common in HL, thoracic CT was reported to be more sensitive than 18F-FDG PET in the detection of pulmonary lesions in HL patients (70% vs. 100%) (11). Detection of bone marrow involvement is important, as it upstages the patient to stage IV disease, which changes prognosis and management. Bone marrow biopsy is used for clinical staging decisions but obtains information from a limited area, typically the iliac crest. 18F-FDG PET/CT provides information about the entire bone marrow, beyond clinical biopsy areas (Table 2) (35–37). Several authors reported that 18F-FDG PET/CT accurately detected bone marrow involvement outside the pelvis in patients with negative results from routine biopsies of the iliac crest (35–38) and have suggested that 18F-FDG PET/CT can supplant marrow biopsy for purposes of staging.
Diagnostic Value of 18F-FDG PET and 18F-FDG PET/CT for Staging and Restaging of Pediatric Patients with Malignant Lymphoma
A 10-y-old girl with HL demonstrating multiple 18F-FDG–avid bone metastases (arrows, A and B; SUVmax, 14.9) in thoracic spine that are not detectable on dedicated diagnostic CT (C) and radionuclide bone scanning (D).
Diagnostic Value of 18F-FDG PET and 18F-FDG PET/CT for Detection of Bone Marrow Involvement in Pediatric Patients with Malignant Lymphoma
Therapy Response Assessment
Standard-of-care treatment of pediatric HL typically involves chemotherapy, which may be followed by involved-field radiotherapy in some patients. Patients with early-stage HL often do not receive radiotherapy, whereas those with intermediate and advanced stages have demonstrated improved progression-free survival with radiotherapy (39). Tumor response to chemotherapy is used as a criterion to determine the need for radiotherapy and the radiation dose. If radiotherapy is necessary, the radiation field is planned according to the initial extent of disease. Patients with NHL represent a heterogeneous group, which is typically treated with chemotherapy. The use of radiotherapy is limited in pediatric NHL (40,41).
Several investigators reported, for both pediatric HL and pediatric NHL, that additional information obtained on 18F-FDG PET scans compared with CT scans changed management in up to 32% of patients (20,42,43). The most frequent management impact was avoiding radiotherapy of soft-tissue masses in HL, which did not show significant hypermetabolism on 18F-FDG PET scans at the end of therapy. In 17% of advanced-stage HL, additional disease sites detected on 18F-FDG PET/CT compared with CT alone resulted in an extended radiation field (33).
It is important to identify nonresponders early, to avoid ineffective treatments and enable early stratification to intensified or alternative treatment options. Likewise, correct identification of patients who could be spared from radiotherapy is important to avoid radiation-related complications. In both pediatric HL and pediatric NHL, the level of 18F-FDG tumor uptake on interim 18F-FDG PET/CT scans after initiation of chemotherapy has shown higher accuracy for therapy response assessment than evaluations of changes in tumor size on conventional imaging (14,25,27,44). However, interim 18F-FDG PET/CT scans after 2–3 cycles of standard chemotherapy showed a relatively wide range of sensitivity (77.8%–100%) and specificity (54.5%–97.7%) (Table 1) (26,38,45,46). This may be due to lack of consensus on the best timing of interim PET and the definition of objective response criteria for interpretation (47–49). Nevertheless, interim 18F-FDG PET/CT has shown high negative predictive value, and therefore an early negative scan is a reliable indicator for therapy response (negative predictive value, 85.7%–100%; positive predictive value, 41.2%–85.7%) (26,38,45). In HL patients, Furth et al. reported that a negative interim 18F-FDG PET/CT scan after 2 cycles of chemotherapy is a strong indicator of relapse-free survival, with a negative predictive value of 100% (46). Therefore, an interim 18F-FDG PET/CT scan has been advocated by many investigators and has led to early intensification of chemotherapy in apparent nonresponders (46,50,51). A substantial fraction (≤65%) of patients with positive interim PET results will still be cured, and patients with negative or positive interim results seem to do well if their PET results are negative at the completion of chemotherapy (typically 6 cycles) (52). Therefore, other investigators suggest performing follow-up 18F-FDG PET/CT scans at later time points (53).
In NHL patients, Yang et al. reported that a persistent tumor 18F-FDG uptake on interim 18F-FDG PET/CT scans predicted worse overall survival and event-free survival (54). However, this principle may not hold for all types of NHL (38,43). A recent study on nonlymphoblastic lymphoma patients showed that neither interim 18F-FDG PET/CT nor interim CT scans could predict survival (38).
Reported sensitivities and specificities of 18F-FDG PET/CT for therapy response assessment of HL and NHL at 2 wk to 3 mo after completion of therapy showed wide ranges of 75%–100% and 75%–90.9%, respectively (Table 1) (26,38,46,50,51). More systematic data evaluations are needed to determine the best time point for interim scans for response assessment of pediatric lymphomas.
Information about the value of 18F-FDG PET or 18F-FDG PET/CT follow-up studies of pediatric HL and NHL after therapy is based on few nonresponders per evaluated study population (14,25,43,46,50,55–57). 18F-FDG PET/CT has shown high sensitivity and specificity for the diagnosis of disease relapse in HL and NHL (95%–100% and 90%–100%, respectively) (Table 1) (26,43,56). However, false-positives were noted because of thymic rebound, inflamed lymph nodes, physiologic cardiac uptake (43), infections or inflammation (56), and reconverted marrow. This is a typical false-positive paradox, that is, false-positive results are more probable than true-positive when the overall population has a low incidence of a condition. Therefore, a negative follow-up 18F-FDG PET scan is a strong indicator of absence of disease relapse, whereas a positive scan should be validated with other imaging modalities or biopsy (Fig. 2) (56).
(A) A 14-y-old boy with multiple intensely 18F-FDG–avid mediastinal lymph nodes on contrast-enhanced PET/CT consistent with HL at time of staging (SUVmax, 4.14). (B) Posttherapy contrast-enhanced 18F-FDG PET/CT demonstrates persistent lymphadenopathy on CT (arrows). Lymphadenopathy is negative for 18F-FDG activity, consistent with complete response to therapy. Patient achieved progression-free survival at 2 y follow-up.
Several recent studies have demonstrated that routine follow-up by 18F-FDG PET/CT and other imaging techniques may be overused for routine surveillance of patients with HL, contributing to increased cost and radiation exposure without a clear survival benefit (6,50,58). More data are needed to determine which patient group will benefit from which surveillance test for how long and at which frequency. For example, Burkitt lymphoma nearly always recurs within the first year after treatment, whereas HL typically recurs within the first 2 y (58–60). Favorable prognostic indicators may allow limiting or omitting follow-up imaging. For example, patients with low-risk HL and negative 18F-FDG PET results at the end of therapy do not require further imaging unless relapse is clinically suspected (61–63). Current guidelines for HL do not recommend routine follow-up 18F-FDG PET scans for HL patients (64,65).
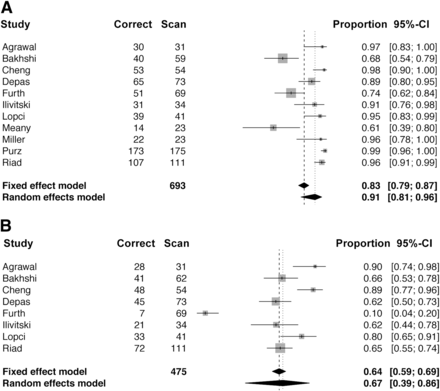
Tables 3 and 4 provide information about sensitivity, specificity, and accuracy of 18F-FDG PET or PET/CT, as well as conventional imaging modalities, for staging or therapy response assessment of malignant lymphomas. The high degree of variability among these studies did not provide an adequate sample size for an inferential metaanalysis. However, the pooled data of 11 articles allowed an estimate of the per-modality accuracy (Fig. 3). Although the 95% confidence intervals for the 2 modalities overlap, the random-effects estimates of accuracy strongly suggest that PET is at least as accurate as, if not more accurate than, conventional imaging modalities (91% vs. 67%). Studies with large populations of pediatric patients are needed to confirm the additive value of PET.
Characteristics of Studies Eligible for Metaanalysis
Diagnostic Accuracy of 18F-FDG PET and PET/CT in Studies Eligible for Metaanalysis
Overall diagnostic accuracy of 18F-FDG PET or PET/CT (A) and conventional modalities (B) in pediatric lymphoma patients. Three studies reported only PET data.
BONE AND SOFT-TISSUE SARCOMAS
Bone and soft-tissue sarcomas account for 10% of all childhood malignancies and 8% of malignancies in adolescents and young adults (66), with an annual incidence of approximately 2 per 100,000 patients (67). The most common soft-tissue sarcomas in patients less than 20 y old are rhabdomyosarcomas (60% of soft-tissue sarcomas) (1,68), with an incidence of 4.5 cases per million per year (68). Fibrosarcomas, synovial sarcomas, and extraosseous Ewing sarcomas represent other soft-tissue sarcomas in the pediatric population (69). The most common bone sarcomas in children and adolescents are osteosarcomas and Ewing sarcoma family tumors, which comprise Ewing sarcoma of the bone, extraosseous Ewing sarcoma, primitive neuroectodermal tumors, neuroepithelioma, and Askin tumor (70).
Initial Staging
Most studies that evaluated 18F-FDG PET/CT for staging of bone and soft-tissue sarcomas in children were based on small cohorts and heterogeneous collections of tumors (Table 5). The presence of metastases is common in sarcomas and is associated with poor prognosis (71,72). Typical sites to be investigated with imaging tests are lymph nodes (more common in rhabdomyosarcomas, less common in bone sarcomas), lung, and bone (73–75). Nearly any other organ, such as brain, liver, and pancreas, can be affected as well (73,76). Tateishi et al. reported that in 16% of patients, 18F-FDG PET/CT detected distant metastases that could not be detected with either conventional modalities or PET alone (77). The sensitivity and specificity of 18F-FDG PET/CT for staging of all sarcomas ranged from 85.7%–100% and 97%–100%, respectively, for detection of nodal lesions to 56%–100% and 91%–100%, respectively, for detection of distant metastasis, including pulmonary lesions (Table 5) (71,77–79). For initial staging of rhabdomyosarcomas, the reported sensitivities and specificities of 18F-FDG PET/CT were 93.8%–100% and 100%, respectively, for detection of nodal lesions and 100% and 91%, respectively, for detection of distant metastases (Fig. 4A) (71,79). In cases of bone sarcomas, 18F-FDG PET/CT is more sensitive for the detection of extrapulmonary metastases (83.3% vs 77.8%) and more specific (98.1% vs. 96.7%) than conventional imaging modalities (3).
Diagnostic Value of 18F-FDG PET and 18F-FDG PET/CT for Staging and Restaging of Pediatric Patients with Malignant Sarcomas
(A) An 11-y-old girl with 18F-FDG–avid rhabdomyosarcoma identified on staging 18F-FDG PET/CT in soft tissue overlying right maxilla, with SUVmax of 5.4. (B) Unexpected right popliteal lymph node metastasis discovered on staging whole-body 18F-FDG PET/CT in transaxial plane (red arrow; SUVmax, 3.6). (C) Posttherapy 18F-FDG PET/CT demonstrating resolution of 18F-FDG activity in facial tumor (red arrows). (D) 18F-FDG PET/CT showing new abnormal activity (yellow arrows; SUVmax, 2.2) consistent with recurrent tumor, confirmed by biopsy.
Pulmonary Metastases
Chest CT detected pulmonary metastases with higher sensitivity (93.3%–100%) than 18F-FDG PET (25%) (3,78) because of the increased anatomic resolution of CT for detection of subcentimeter pulmonary nodules and variable 18F-FDG uptake in pulmonary nodules (2,3).
18F-FDG PET showed higher specificity in detecting malignant pulmonary nodules (95.8%) than did conventional imaging, including chest CT (87.3%) (Table 6) (3). Because the default clinical intervention for pulmonary nodules in sarcoma patients is surgical resection, it would be important to generate more reliable imaging indicators for exclusion of malignancy, which could avoid unnecessary surgeries of granulomas, intrapulmonary lymph nodes, and other benign lesions. Future studies should aim to ascertain specific imaging characteristics of benign lesions.
Diagnostic Value of 18F-FDG PET and 18F-FDG PET/CT for Detection of Pulmonary Metastases in Pediatric Patients with Malignant Sarcomas
Bone Metastases
The detection of bone metastases significantly affects overall survival. For example, overall 3-y event-free survival in patients with metastatic rhabdomyosarcomas is 32% in the absence of bone metastases and only 15% in the presence of bone metastases (80).
Several studies on heterogeneous patient populations with bone and soft-tissue sarcomas demonstrated that in the detection of bone lesions, sensitivity and diagnostic accuracy were higher for 18F-FDG PET/CT than for 99mTc-methylene diphosphonate bone scans (81–84) and conventional imaging (radiography, CT, MR imaging, and ultrasonography) (78). It was recommended that bone scans be omitted if bone sarcomas are evaluated with 18F-FDG PET (81). The appropriateness of this recommendation was confirmed by studies that evaluated rhabdomyosarcomas and bone sarcomas separately: for detection of bone metastases in rhabdomyosarcomas, 18F-FDG PET/CT showed a sensitivity of 100%, compared with 66% for conventional imaging modalities, including radiography, CT, MR imaging (79), or bone scintigraphy alone (2,71). For detection of bone metastases in Ewing sarcomas, 18F-FDG PET showed a sensitivity of 88%, compared with 37% for conventional imaging modalities (78).
In osteosarcomas, however, the sensitivities of 18F-FDG PET and conventional imaging modalities (radiography, CT, MR imaging, and bone scintigraphy) for bone lesion detection were equal (90% for both) (78). The sensitivity of 18F-FDG PET was only slightly higher than bone scintigraphy (90% vs. 81%), and this difference was not statistically significant (78). A likely explanation could be the higher osteoblastic activity in osteosarcoma metastases, as opposed to the predominant bone marrow infiltration in Ewing sarcomas (78,83,85).
Lymph Node Metastases
The presence of regional lymph node metastases is a strong prognostic factor in rhabdomyosarcoma patients (72,86). 18F-FDG PET/CT was more sensitive (94%–100%) and more accurate (95%–100%) than conventional imaging (75%–94% and 49%–88%, respectively) for detection of lymph node metastases (71,79). Integrated 18F-FDG PET/CT was also more accurate than 18F-FDG PET alone (96% vs. 86%) (77). Ricard et al. reported that 18F-FDG PET/CT changed lymph node staging in 4 of 13 rhabdomyosarcoma patients by downstaging 1 patient and detecting lymph node involvement not shown by conventional imaging modalities in 3 patients (2).
The low incidence of lymph node metastases in patients with osteosarcoma and Ewing sarcoma family tumors carries a risk of false-positive findings, since inflammatory lymph nodes with a high maximum standardized uptake value (SUVmax) are relatively more common. Thus, interventions are needed to decrease 18F-FDG uptake by inflammatory nodes (and thereby decrease the incidence of false-positives), perhaps through antiinflammatory treatment before a staging 18F-FDG PET/CT scan.
Prognostic Value
18F-FDG PET/CT can predict survival in pediatric sarcoma patients based on the metabolic activity of the primary tumor at the time of initial diagnosis (1,87,88). In a study on 41 rhabdomyosarcoma patients, hypermetabolism of the primary tumor (SUVmax/SUVliver > 4.6) or metastases was linked to lower survival rates (1). In the same cohort, 44% of patients with high-intensity primary tumors (18F-FDG uptake close to brain activity) at initial diagnosis died within 49 mo. By contrast, all patients with low-intensity primary tumors (18F-FDG uptake ≤ liver activity) survived (1). Only 44% of patients with metabolically active lymph nodes and 50% of patients with hypermetabolic metastases survived (1).
Therapy Response Assessment
Neoadjuvant and adjuvant chemotherapy increased survival rates in sarcoma patients (89–91). Therapy response in clinical practice is currently assessed on the basis of change in the longest tumor diameter for soft-tissue sarcomas (Response Evaluation Criteria in Solid Tumors) and 3-dimensional changes in tumor size for bone sarcomas (Children’s Oncology Group criteria). Metastases are evaluated by the same criteria if they are larger than 10 mm. A reduction in the soft-tissue component of bone sarcomas in response to chemotherapy does not necessarily predict a favorable outcome (92). Therefore, additional imaging biomarkers are needed to identify patients with poor outcomes and stratify these patients to more aggressive therapies.
There is some evidence that the degree of 18F-FDG uptake on PET scanning may provide additional information for therapy response assessment, compared with morphologic assessments on CT and MR imaging scans (Table 7) (92–94).
Assessment of Chemotherapy Response of Malignant Sarcomas in Pediatric Patients with 18F-FDG PET/CT
In osteosarcomas, Denecke et al. reported that an overall tumor SUV and SUVmax on posttreatment 18F-FDG PET/CT scans were more accurate for the assessment of therapy response than changes in tumor volume (92). Using a cutoff SUVmax of less than 2.8, 18F-FDG PET/CT differentiated responders from nonresponders with up to 100% accuracy after completion of neoadjuvant therapy (92,93). Im et al. even reported that an interim 18F-FDG PET/CT scan after only a single course of neoadjuvant chemotherapy is useful in predicting tumor response (94). Histopathologic tumor response was associated with a median decrease of 75% in SUVmax, whereas nonresponders had a significantly lower decrease of 41% (92).
In Ewing sarcomas, 18F-FDG PET/CT could not accurately predict therapy response, because both responders and nonresponders showed decreased 18F-FDG tumor uptake (92). The predictive value of response on imaging tests is highly dependent on timing. In general, a patient who is responding well early after the initiation of therapy is likely to do well. As therapy continues, more patients will have responded and the test loses its predictive ability. For example, in acute lymphoblastic leukemia, 98% of patients will enter complete remission after 4 wk of chemotherapy, yet 20% will relapse. Thus, the assessment at 4 wk does not predict outcome very well (except for the 2% who do not reach any remission). Conversely, response assessment at 1 or 2 wk into therapy, when a smaller proportion of patients has attained remission, is more informative. Therefore, future studies should evaluate earlier 18F-FDG PET assessments of response in Ewing sarcomas. In rhabdomyosarcomas, 18F-FDG PET/CT correctly identified tumor response in 92% of patients after 3 cycles of chemotherapy, compared with 84% with conventional modalities (including chest radiography, bone marrow biopsy, contrast-enhanced CT, and contrast-enhanced MR imaging) (79).
Information about the value of 18F-FDG PET/CT for the detection of tumor recurrence is limited. Arush et al. reported a high accuracy of 18F-FDG PET/CT in the detection of local recurrence (95%) of Ewing sarcomas, osteosarcomas, and rhabdomyosarcomas on follow-up scans (Fig. 4B) (95). In the case of a new soft-tissue lesion on conventional imaging scans, which is of low suspicion for residual or recurrent disease, an 18F-FDG PET/CT examination may be reassuring to exclude disease and avoid biopsy. In the case of any suggestive lesion, biopsy is warranted, and if the biopsy result is positive, 18F-FDG PET/CT may be useful for restaging for additional tumor sites. More evidence is needed on diagnostic algorithms for the detection of tumor recurrence.
NEUROBLASTOMAS
Neuroblastoma is the most common pediatric extracranial soft-tissue tumor, accounting for 8% of all childhood malignancies (96). Its annual incidence is about 10.5 cases per 1 million children (97). Metastases to lymph nodes, liver, bone, and bone marrow are frequent in neuroblastoma patients, but nearly any other organ can be affected as well (98). 123I- or 131I-labeled metaiodobenzylguanidine (MIBG) is used as a biomarker for clinical whole-body staging and restaging with planar scintigraphy and SPECT (96,99,100). About 10% of neuroblastomas do not show MIBG uptake. In these patients, bone scans and 18F-FDG PET scans were useful additional tools for whole-body staging (96,99,101).
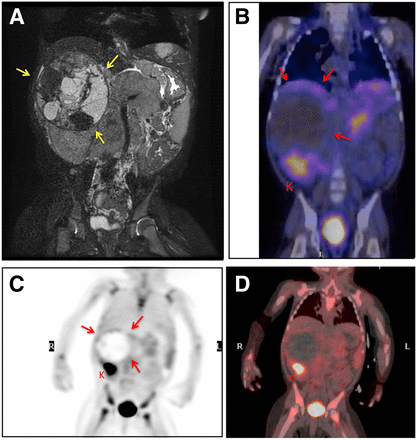
Both MIBG-positive and MIBG-negative neuroblastomas demonstrated moderate 18F-FDG uptake (Fig. 5) (10,102). The SUVmax of primary tumors was lower in early-stage (I–II) than advanced (III–IV) neuroblastoma (3.03 vs. 5.45, respectively, P = 0.019) (103). The inferior diagnostic accuracy of 18F-FDG for the detection of distant metastases was mainly due to superior tumor-to-background contrast (10) and improved detection of bone and bone marrow disease with MIBG scintigraphy (104). The high metabolic activity of normal hematopoietic bone marrow in young children masked metastases on 18F-FDG PET scans (104). Therefore, 123I-MIBG scintigraphy was reported to be superior to 18F-FDG PET in stage 4 neuroblastomas (104). Also, calvarial metastases in neuroblastoma patients may be masked by adjacent intense physiologic brain activity on 18F-FDG PET scans (105). In another study, which primarily evaluated patients with advanced disease (no cranial vault lesions) after primary tumor resection and chemotherapy, MIBG scintigraphy and 18F-FDG PET were equally effective in detecting bone metastases (106). This finding can be explained by the decreased metabolic activity of normal bone marrow after chemotherapy. However, other authors found that 18F-FDG PET was problematic after granulocyte colony-stimulating factor therapy because of the associated increased bone marrow uptake (104).
(A) A 2-y-old girl with large cystic abdominal neuroblastoma identified on coronal T2-weighted contrast MR imaging (yellow arrows) at time of diagnosis. (B–D) Pretherapy 123I-MIBG-SPECT/CT (B), 18F-FDG PET (C), and 18F-FDG PET/CT (D) demonstrate subtle mild activity along rim of tumor (red arrows; SUVmax, 3.3). Patient had chemotherapy-related diffuse bone marrow activity. K = normal kidney.
Choi et al., in a study of 30 neuroblastoma patients, found that 18F-FDG PET is more sensitive than CT in the evaluation of distant lymph node involvement and can help in detecting recurrent lymph node metastases (103). Therefore, 18F-FDG PET/CT might be particularly helpful in older patients who present with small, resectable primary tumors and chronic lymph node metastases.
New radiotracers such as 124I-MIBG PET (107), 18F-DOPA (108), and 18F-MIBG (109) may be the future for neuroblastoma staging and treatment monitoring. These tracers may obviate 24-h follow-up scans after tracer injection and enable theranostic approaches with combined diagnostic and therapeutic tracers.
WILMS TUMOR
Wilms tumor is the most common renal tumor in children, accounting for 6% of all pediatric malignancies and having an annual incidence of 500 cases in the United States. The overall 5-y survival rate exceeds 90% (110). Important information for local tumor staging such as tumor location, size, local extension, vascular compression or invasion, and local lymph node metastases is best evaluated with CT, MR imaging, and ultrasonography (111). The most common site for metastases is the lungs, which are typically evaluated with chest CT (111). Therefore, 18F-FDG PET is of limited value for assessment of Wilms tumors.
Wilms tumors show strong 18F-FDG uptake (112,113), with a reported SUVmean of 6.8 (113). Anaplastic Wilms tumors demonstrated particularly high 18F-FDG uptake, with an SUVmax greater than 5, because of highly expressed glucose transporter 1 (112). There was otherwise no significant relation between 18F-FDG uptake and tumor histopathology (111).
There are 2 major management approaches for Wilms tumor: one prefers initial surgery followed by chemotherapy, and the other prefers neoadjuvant chemotherapy followed by surgery (114). In patients who underwent preoperative systemic therapy, Misch et al. could not find any correlation between 18F-FDG PET parameters (SUV reduction, initial SUV, posttherapy SUV, visual interpretation) and morphologic response assessments (volume reduction obtained by CT or MR imaging) (113). Conversely, Begent et al. reported lower tumor SUVs after chemotherapy in tumors that did respond to presurgical chemotherapy compared with those that did not (112). The differentiation between Wilms tumors and other kidney tumors would provide important information for patient management. To the best of our knowledge, no systematic studies exist that compare the degree and distribution of 18F-FDG uptake in Wilms tumor, nephroblastomatosis, clear cell sarcoma, rhabdoid tumor, and renal cell carcinoma, among others. A study that could differentiate between Wilms tumor and nephroblastomatosis would be particularly clinically significant.
BRAIN TUMORS
Brain tumors are the most common solid tumors in children, accounting for 20%–25% of all childhood malignancies and having an annual incidence of nearly 2,200 cases (115,116). The most common brain tumors in pediatric patients are pilocytic astrocytomas (26.2% of childhood brain tumors) and primitive neuroectodermal tumors or medulloblastomas (21.9% of cases). Other types are gliomas (19.4%), ependymomas (7.8%), other astrocytomas (12.3%), glioblastomas (3.5%), oligodendrogliomas (1.9%), and others (0.7%) (117).
18F-FDG PET/CT is not routinely used for clinical evaluation of pediatric brain tumors because the physiologic high 18F-FDG uptake of the normal brain limits tumor detection, especially in low-grade gliomas (118). However, 18F-FDG PET/CT may provide prognostic information based on the tumor’s metabolic activity: in children with anaplastic astrocytomas, the degree of 18F-FDG uptake was positively correlated with histopathologic tumor grade (119) and correlated with progression-free survival (120). In children with low-grade astrocytomas, progressive disease showed increased 18F-FDG uptake, and initial hypermetabolism correlated with a shorter interval to progression (33 wk vs. 52.3 wk) (121). In children with brain stem gliomas, survival rates were significantly decreased when more than 50% of the tumor was 18F-FDG–avid (122). Some benign tumors also demonstrate high 18F-FDG uptake; these include juvenile pilocytic astrocytomas (123), choroid plexus papilloma (124), and pleomorphic xanthoastrocytoma (125), among others. More specific tracers that can better differentiate benign from malignant brain tumors, such as new amino acid tracers, choline analogs, or radiolabeled nucleoside analogs, are critically needed (119). Pirotte et al. suggested that absence of 18F-FDG PET tumor uptake might justify more conservative treatment (126).
Evaluation of response to chemotherapy with 18F-FDG PET is complicated in pediatric brain tumors because corticosteroids, chemotherapy, and radiation therapy all change cerebral glucose uptake (127,128). Interestingly, tumor metabolic activity, diagnosed with 18F-FDG PET and MR spectroscopy, provide complementary information (129). The introduction of integrated PET/MR scanners may enable more detailed investigations of the clinical value of 18F-FDG PET imaging in pediatric brain tumors. Non–18F-FDG radiotracers such as 3′-deoxy-3′-18F-fluorothymidine or 18F-DOPA are promising tools for pediatric brain tumor imaging (130,131).
CONCLUSION
18F-FDG PET/CT provides important information for staging and restaging of malignant lymphomas and other tumors in pediatric patients. However, the cumulative ionizing radiation dose caused by repeated 18F-FDG PET/CT scans is a major concern for children. To address this issue, several guidelines have recently been developed for low-dose 18F-FDG PET/CT protocols, involving injections of reduced radiotracer activity, low-dose CT protocols, and integration of CT scans for attenuation correction and diagnostic purposes.
For tumor staging of pediatric patients, recent developments in whole-body MR imaging and PET/MR provide alternatives with substantially reduced or even eliminated radiation exposure. Diagnostic accuracies, costs, and the clinical impact of these new technologies have to be tested against established PET/CT benchmarks. As we develop whole-body imaging technologies further, it is important to not simply duplicate PET/CT approaches on different (and potentially more expensive) technologic pieces of equipment. We have to avoid reinventing the wheel by “discovering” advantages that have been previously identified on PET/CT studies. We hope that this article has helped to elucidate established concepts of PET and PET/CT technologies for pediatric cancer staging such that we can proceed to address unresolved questions, truly advancing this technology and making an impact on the lives of our patients.
Footnotes
Published online Jan. 8, 2015.
Learning Objectives: On successful completion of this activity, participants should be able to (1) understand the current status of the role of 18F-FDG PET and PET/CT for staging and treatment monitoring of pediatric malignancies; (2) discuss the limitations of 18F-FDG PET and PET/CT for pediatric cancer imaging; and (3) discuss areas of pediatric cancer imaging in which further studies are needed.
Financial Disclosure: The authors acknowledge support from the Child Health Research Institute, Stanford University. Dr. Uslu was a resident at the Department of Nuclear Medicine at Istanbul University Cerrahpasa Medical Faculty, Istanbul, Turkey. She was supported as a visiting scholar by the Scientific and Technological Research Council of Turkey (TÜBİTAK). The authors of this article have indicated no other relevant relationships that could be perceived as a real or apparent conflict of interest.
CME Credit: SNMMI is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to sponsor continuing education for physicians. SNMMI designates each JNM continuing education article for a maximum of 2.0 AMA PRA Category 1 Credits. Physicians should claim only credit commensurate with the extent of their participation in the activity. For CE credit, SAM, and other credit types, participants can access this activity through the SNMMI website (http://www.snmmilearningcenter.org) through February 2015.
- © 2015 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.
- 60.↵
- 61.↵
- 62.
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.↵
- 90.
- 91.↵
- 92.↵
- 93.↵
- 94.↵
- 95.↵
- 96.↵
- 97.↵
- 98.↵
- 99.↵
- 100.↵
- 101.↵
- 102.↵
- 103.↵
- 104.↵
- 105.↵
- 106.↵
- 107.↵
- 108.↵
- 109.↵
- 110.↵
- 111.↵
- 112.↵
- 113.↵
- 114.↵
- 115.↵
- 116.↵
- 117.↵
- 118.↵
- 119.↵
- 120.↵
- 121.↵
- 122.↵
- 123.↵
- 124.↵
- 125.↵
- 126.↵
- 127.↵
- 128.↵
- 129.↵
- 130.↵
- 131.↵
- 132.
- 133.
- 134.
- Received for publication August 25, 2014.
- Accepted for publication December 2, 2014.