Abstract
18F-FDG PET/CT has been proven to be a highly sensitive method for pheochromocytomas/paragangliomas (PHEOs/PGLs) associated with succinate dehydrogenase (SDH) mutations. This finding has been attributed to altered tumor cell metabolism resulting from these mutations and does not provide additional prognostic information to genotype. Therefore, identification of new biomarkers for aggressiveness is needed. A high Ki-67 index was proposed to be an additional prognostic factor. This pilot study aimed to evaluate 3′-deoxy-3′-18F-fluorothymidine (18F-FLT) PET/CT, a PET proliferation tracer, as a potential imaging agent in a series of 12 PHEO/PGL patients with different genetic backgrounds, to compare 18F-FLT uptake with 18F-FDG PET/CT, and to evaluate classic factors of aggressiveness. Methods: Twelve patients (7 metastatic and 5 nonmetastatic) were prospectively evaluated with 18F-FDG and 18F-FLT and followed for at least 2 y after the initial imaging work-up. Uptake was assessed at a lesion level, visually and quantitatively by maximum standardized uptake values (SUVmax) for both tracers. 18F-FLT uptake was compared with risk factors known to be linked with a poor prognosis in PGLs (SDHB-mutated status, lesion size, dopaminergic phenotype) and with 18F-FDG uptake. Results: In 12 patients, 77 lesions were assessed. All lesions had low 18F-FLT uptake (median SUVmax, 2.25; range, 0.7–4.5). There was no apparent superiority of 18F-FLT uptake in progressive lesions, and most of the lesions showed a mismatch, with high 18F-FDG uptake (median SUVmax, 10.8; range, 1.1–79.0) contrasting with low 18F-FLT uptake. Conclusion: This study suggests that PHEOs/PGLs—even those that progress—do not exhibit intense 18F-FLT uptake. It provides the first in vivo demonstration that proliferation may not be a major determinant of 18F-FDG uptake in these tumors. These findings provide new insight into the biologic behavior of PGL and suggest that antiproliferative agents may be suboptimal for treatment of these tumors.
- pheochromocytoma and paraganglioma
- 18F-fluorothymidine
- 18F-fluorodeoxyglucose
- positron emission tomography
- proliferation
- glycolysis
Pheochromocytomas (PHEOs) and extraadrenal paragangliomas (PGLs) are neural crest cell–derived tumors associated with either the sympathetic (thoracoabdominal PGLs) or the parasympathetic (mainly head and neck PGLs) nervous systems.
Approximately 40% of PHEOs and PGLs carry a germline mutation in 1 of at least 18 genes (1). These mutations are associated with transcriptome changes that are currently subdivided into 2 main clusters. Cluster 1 is enriched with genes (i.e., succinate dehydrogenase complex, subunits A-D [SDHx]) that are associated with the hypoxic response (mainly hypoxia-inducible factor [HIF]-2α), and cluster 2 contains tumors mutated for genes that activate kinase signaling and protein translation (i.e., RET protooncogene-MEN2) (2).
Overall, the malignancy risk for PHEOs/PGLs has been estimated to be 10%, with an increased risk in sympathetic PGLs belonging to the cluster 1 subgroup.
At the present time, there are no reliable cytologic, histologic, immunohistochemical, molecular, or imaging criteria for determining malignancy (3). The diagnosis of malignancy remains strictly based on the finding of metastases where paraganglial cells are not usually present, such as the lymph nodes, lung, bone, or liver. To this end, anatomic and functional imaging play a central role in ruling out metastases but are still limited in that they cannot provide further information about the potential behavior (e.g., malignant potential, proliferation rate, degree of apoptosis, and hypoxia) of these tumors that is closely linked to their genotype, biochemical properties, and localization.
Beyond SDHB mutation status, classic indicators of poor prognosis include a tumor size greater than 5 cm, tumor location (extraadrenal), age younger than 30 y at first presentation, and metastatic disease (4–6). Recently, some new studies have proposed the prediction of metastatic potential or tumor aggressiveness using characteristics such as dopaminergic phenotype (i.e., detection of dopamine or its metabolite methoxytyramine) (7,8), the presence of tumor necrosis, high Ki-67 index or mitotic count (9), overexpression of HIF-α and its target genes in tumors (10,11), or extremely high messenger RNA copy numbers of a variant of carboxypeptidase E in tumors (12). Thus, the identification of in vivo biomarkers of aggressiveness would be of particular interest in the assessment of these tumors.
Interestingly, PHEOs/PGLs belonging to the cluster 1 exhibit increased 18F-FDG uptake on PET/CT, compared with cluster 2 tumors (13). 18F-FDG PET/CT has been proven to provide prognostic information in several endocrine cancers such as thyroid carcinomas of follicular origin (14), medullary thyroid carcinoma (15), and gastroenteropancreatic neuroendocrine tumors (16). In these tumors, acquisition of the 18F-FDG metabolic pattern is progressive during the dedifferentiation process. In contrast, 18F-FDG PET/CT avidity was found to be mainly dependent on the presence of a pseudohypoxic phenotype (activation of the HIF-signaling pathway despite normal oxygen supply) in PHEOs/PGLs, a finding that is in large part dependent on the genetic background (cluster 1 genes) (2,13,17,18). Therefore, the 18F-FDG PET/CT avidness does not provide prognostic information in PHEOs/PGLs, and SDHx tumors may have an indolent course, even with highly elevated uptake values of 18F-FDG.
3′-deoxy-3′-18F-fluorothymidine (18F-FLT) has been proposed as a PET proliferation tracer even though it is not incorporated into DNA because of phosphorylation by cytosolic thymidine kinase-1. The assumption is that the concentration of 18F-FLT nucleotides in cells is proportional to thymidine kinase-1 activity and, therefore, to cellular proliferation. The role of 18F-FLT in oncology is still debated, but several studies have shown promising results for tumor grading and in the evaluation of treatment response (19).
The aims of the present study were to evaluate 18F-FLT PET/CT in a series of 12 PHEO/PGL patients with varying genetic backgrounds and to evaluate the relationships between 18F-FLT uptake and classic factors of aggressiveness (e.g., presence of SDHB mutation, age ≤ 30 y at diagnosis, metastatic disease, large lesions [>5 cm], and elevated dopamine secretion). 18F-FDG PET/CT was also performed with head-to-head comparison between 18F-FDG and 18F-FLT lesion uptake in order to better understand the relationship between glycolysis and cell proliferation in these tumors.
MATERIALS AND METHODS
Patients
Twelve nonconsecutive adult patients (10 men and 2 women; median age, 43 y; age range, 27–70 y) with PGLs (as defined by the reference standard) were prospectively included between January and July 2012 (and followed up over the course of at least 2 y). All patients were studied at the National Institutes of Health. The study protocol (00-CH-0093) was approved by the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, and all subjects provided written informed consent. The inclusion criterion was to have at least 1 PGL (as defined by the reference standard) at the time of the study. Exclusion criteria included age younger than 18 y, pregnancy, or recent (<2 mo) systemic treatment.
Reference Standard to Define PGL
PGL lesions were confirmed histologically when surgery was performed on patients with nonmetastatic disease (patients 1, 2, 5, 8, and 9). When surgery was not indicated because of the presence of metastases (patients 3, 4, 6, 7, 10, 11, and 12), lesions were characterized as PGL-related (either primary or metastatic) based on their positivity (characteristic appearance) on conventional imaging—either contrast-enhanced CT or contrast-enhanced MR imaging—that corresponded with positivity on either 6-18F-fluoro-3,4-dihydroxyphenylalanine (18F-FDOPA) or 6-18F-fluorodopamine (18F-FDA) PET/CT (13,20,21); these findings were further supported by positive PGL-specific biochemistry and genetic testing results.
The following data were collected: metastatic status (defined by the presence of tumor cells at nonneural crest–derived sites), genetic testing, adrenal versus extraadrenal location, tumor size (patient carrying at least 1 tumor diameter > 5 cm, which is the optimal cutoff from Eisenhofer’s analysis (8)), dopaminergic phenotype (i.e., presence of dopamine or its metabolite methoxytyramine in urine or plasma (8)), and age at diagnosis. Patients were followed (clinically, biochemically, and with imaging) for at least 2 y after their initial imaging.
Imaging Protocol for 18F-FDG and 18F-FLT PET/CT Scans
All patients underwent 18F-FDG and 18F-FLT PET/CT scanning. 18F-FLT was provided by Cardinal Health. A fixed 18F-FLT dose of 195 MBq was injected. For 18F-FDG PET scanning, patients fasted for at least 4 h before intravenous injection of 18F-FDG (5 MBq/kg; median total injected dose, 390 MBq; range, 340–590 MBq), and blood glucose levels were measured just before injection to ensure a value below 200 mg/dL. For both radiopharmaceuticals, PET/CT acquisitions were performed on a Biograph-128 mCT PET/CT scanner (Siemens Medical Solutions). For both studies, PET data acquisition started at 60 min after injection. Emission images were obtained in 3-dimensional mode, with 3-min per bed position and 5-min per bed position rates for 18F-FDG and 18F-FLT, respectively. The acquisitions were done from the thighs to the head. Coregistered CT studies for attenuation correction and anatomic coregistration were performed with the following imaging parameters: 120 kV, 115 mA, and a 1.5-mm section thickness. Coregistered images were displayed on a workstation (DeltaViewer; MedImage), with 3-dimensional fused navigation along the axial, coronal, and sagittal planes and maximum-intensity-projection rendering.
18F-FLT and 18F-FDG PET/CT scans were obtained within 1 mo of each other in all but 1 case, with 18F-FLT obtained first in 2 patients (patients 2 and 6) and 18F-FDG performed first in the 10 other patients. For patient 10, the 18F-FLT scan was acquired 4 mo after the 18F-FDG scan.
18F-FDG and 18F-FLT PET Image Analysis
On 18F-FLT and 18F-FDG PET/CT images, uptake was assessed for each lesion (determined on the basis of CT) visually (intensity of uptake in comparison to the surrounding background and to reference organs) and quantitatively. Uptake values were classified according to their location: soft tissue for lesions located in the head and neck area, mediastinum, lung, liver, extrahepatic abdomen and pelvis, and bone. In the case of multiple lesions, a cutoff of 5 lesions per area was fixed.
For quantitative assessment, body weight maximum standardized uptake values (SUVmax) were calculated using the following formula: standardized uptake value (SUV) equals decay-corrected tracer tissue concentration (in Bq/g) injected dose (in Bq) normalized by the patient’s body weight (in g). In both 18F-FLT and 18F-FDG PET, volumes of interest were determined manually over the lesions using coregistered CT. Some small lesions that could not be separated easily from surrounding physiologic uptake (such as in the cases of small liver and bone lesions) were considered nonmeasurable. The largest diameter of each lesion was measured.
Physiologic 18F-FLT uptake was also quantified by SUVmax in the following normal organs: bone marrow (in the L4 vertebral body) and lymphatic tissue (including nonspecific inflammatory lymph nodes, tonsils, and thymic remnants) for use as intrapatient positive controls, and muscle (right paravertebral muscle at the level of L4) for use as an intrapatient nonproliferative negative control. Indeed hematopoietic cells in bone marrow and (to a lesser extent) lymphatic tissue were highly 18F-FLT–positive, as expected for active proliferative tissues (22,23). Liver uptake was also measured.
Histopathologic Analyses and Immunohistochemical Staining
Histopathologic analyses and immunohistochemical staining were performed in 5 patients with nonmetastatic disease in whom tumor tissue was collected prospectively after an 18F-FLT PET scan. Cell and tissue morphology and immunohistochemical staining (chromogranin and synaptophysin) confirmed the diagnosis of PGL, and then Ki-67 levels were assessed. No patient with metastatic disease underwent surgery for ethical reasons: PGL diagnosis could noninvasively be confirmed using imaging, biochemistry, and medical history, and PGL biopsy can lead to severe adverse events related to catecholamine release.
Genetic Testing
Genetic testing included gene sequencing of SDHB, SDHD, RET, and VHL as well as assessment of large gene rearrangements of SDHB and SDHD. When all these were negative, patients were qualified apparently sporadic. Patients were not tested for mutations in the recently described genes SDHA, SDHAF2, and TMEM127.
Statistical Analysis
Descriptive quantitative data were expressed as either a median or given a range. The correlation between 18F-FLT SUV and 18F-FDG SUV was assessed using the Spearman rank correlation coefficient. A P value of less than 0.05 was considered statistically significant.
RESULTS
Patients, Tumors, and Clinical Outcomes
Twelve patients with PHEO/PGL were studied (5 SDHB, 2 SDHD, 1 MEN2, and 4 sporadic). Seven patients were M1, and 5 were M0. Four patients were at the initial stages of PGL disease, 2 were evaluated for recurrence, and 6 for disease progression. One patient had an adrenal PGL, and 11 patients had extraadrenal PGLs. Seven patients had a history of a voluminous (>5 cm) primary PHEO/PGL lesion. Seven patients had previously received antioncologic therapy (131I-metaiodobenzylguanidine in 3 cases; cyclophosphamide, vincristine, and dacarbazine [CVD] chemotherapy in 2 cases; external-beam radiation therapy in 2 cases). Patient characteristics are detailed in Table 1.
Patient Clinical Characteristics
After 18F-FLT PET scanning, 3 patients (patients 10–12) had progressive disease (all associated with the SDHB-mutated genotype, 2/3 with the dopaminergic phenotype). The remaining 9 patients had stable or slowly progressing disease (Table 1).
Seventy-seven lesions were assessed. Of these, 14 lesions were in patients with only primary lesions, and 63 lesions were in patients carrying metastatic lesions. Metastatic sites included soft tissue (lymph nodes) (19), bones (16), liver (10), and lungs (18).
18F-FLT Uptake in PGL Lesions
Of the 77 lesions, 64 were measurable using SUVmax. Thirteen small lesions were nonmeasurable because of surrounding tissue activity: 2 primary PGLs, 6 bone lesions, and 5 liver lesions.
The average tumor 18F-FLT SUVmax was 2.25 (0.7–4.5). 18F-FLT uptake was also observed in normal bone marrow and liver in all cases. In 4 patients (patients 2, 3, 5, and 9), 18F-FLT uptake was noted in 13 lymph nodes/lymphatic tissue–related sites (including tonsils and thymic remnants) thought unrelated to the presence of PHEO/PGL (based on negativity of 18F-FDOPA and 18F-FDA studies). Figure 1 displays the 18F-FLT SUVmax in lesions and reference organs.
18F-FLT SUVmax in lesions and reference organs. PGLs: includes all PGLs (primary tumors only) from patients with metastatic and nonmetastatic disease. Median number of primary PGLs assessed per patient was 3 (range, 1–9). In patients 7, 11, and 12, bone marrow 18F-FLT SUVmax was not measured because of disseminated metastatic bone disease.
18F-FLT in PGL Compared with Clinical Criteria of Poor Prognosis (Per-Patient Analysis)
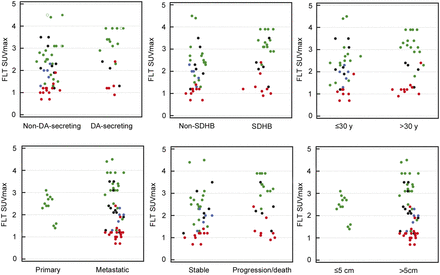
No significant differences in tumor 18F-FLT SUVmax were observed between patients segregated by the presence or absence of poor prognostic factors, including SDHB versus non-SDHB (2.9 [0.9–3.9] vs. 1.9 [0.7–4.5]), age 30 y or younger versus older than 30 y at diagnosis (2.35 [0.9–3.9] vs. 2.05 [0.7–4.5]), metastatic versus nonmetastatic disease (2.05 [0.7–4.5] vs. 2.45 [1.4–3.1]), presence or absence of large (>5 cm) lesions (3.25 [1.3–4.5] vs. 2.45 [1.4–3.1], and elevated versus normal dopamine secretion (median, 2.9 [range, 0.9–3.9] vs. 2.0 [0.7–4.5]). There was also no significant difference in uptake of lesions in patients who had progressive versus stable disease during follow-up (2.4 [0.9–3.9] vs. 2.0 [0.7–4.5]). Figure 2 displays the average 18F-FLT SUVmax in lesions according to these prognostic factors and follow-up.
Lesion 18F-FLT SUVmax compared with criteria of poor prognosis. Green, black, blue, and red refer to primary PGLs, bone metastases, liver metastases, and lung metastases, respectively.
Comparison of 18F-FLT Uptake in PGL with 18F-FDG Uptake
On visual analysis, most lesions (66/77) showed a discrepancy between 18F-FLT and 18F-FDG uptake, with high 18F-FDG uptake contrasting with low or absent 18F-FLT uptake. Figure 3 shows the example of an SDHB patient with a primary abdominal PGL, and Figure 4 is an example of an SDHB patient with widespread metastatic disease.
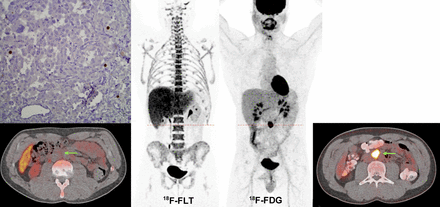
Single retroperitoneal primary PGL in SDHB patient (patient 3). 18F-FDG (maximum-intensity-projection [MIP] image and axial CT–fused image at level of PGL lesion) shows particularly high uptake (18F-FDG SUVmax, 59.8). This lesion corresponded to a 2.6-cm abdominal extraadrenal mass (arrows). 18F-FLT (MIP image and axial CT–fused image at level of PGL lesion) shows low uptake (18F-FLT SUVmax, 2.5). Low Ki-67 staining is demonstrated.
Disseminated metastatic disease (soft tissue, bone, liver, and lung lesions) in SDHB patient. 18F-FDG (maximum-intensity-projection [MIP] image) shows high tumor burden with high 18F-FDG uptake in most of the lesions. 18F-FLT (MIP image) shows low 18F-FLT uptake. Bone lesions (notably in both femoral heads and bilateral iliac bones) appear to have relatively low 18F-FLT uptake compared with surrounding background uptake in marrow.
Overall, the average 18F-FDG SUVmax of the 77 lesions was 10.8 (1.1–79.0), compared with an average SUVmax of 2.25 (0.7–4.5) for 18F-FLT. Correlation analysis demonstrated a weak positive correlation between 18F-FLT and 18F-FDG uptake expressed by SUVmax (Spearman ρ, 0.62; P < 0.05—Fig. 5 shows the scatterplot). Similarly, correlation analyses conducted on subgroups (SDHB-related tumors, non-SDHB–related tumors) demonstrated a weak positive correlation between the 18F-FLT and 18F-FDG SUVmax (Spearman ρ, 0.40, and P = 0.11 for SDHB; and Spearman ρ, 0.59, and P < 0.05 for non-SDHB).
Scatterplot displaying tumor 18F-FLT SUVmax in relation to their 18F-FDG SUVmax. ● = lesions from non-SDHB patients; ○ = lesions from non-SDHB patients. Correlation analysis demonstrated positive correlation between 18F-FLT and 18F-FDG uptake (Spearman ρ, 0.62; P < 0.05).
Ki-67 Immunostaining
In vitro proliferative activity as assessed by Ki-67 index was low (<5%) in all surgically resected lesions (patients 1, 2, 5, 8, and 9). The average 18F-FLT and 18F-FDG SUVmax was, respectively, 2.8 and 6.5 in patient 1, 2.7 and 14.5 in patient 2, 3.1 and 10.4 in patient 5, 2.9 and 53.2 in patient 8, and 3.1 and 46.9 in patient 9.
DISCUSSION
This study shows that PHEOs/PGLs did not exhibit intense 18F-FLT uptake in our 12 patients, even those who progressed rapidly or exhibited high 18F-FDG uptake. It also provides the first, to our knowledge, in vivo demonstration that proliferation is probably not a major determinant of 18F-FDG uptake in these lesions.
These findings differ from highly proliferative cancers (i.e., lymphoma, lung cancer) that exhibit high 8F-FLT tumor uptake values (19). However, the low 18F-FLT uptake found in PGL is concordant with findings observed in well-differentiated gastroenteropancreatic tumors. In 1 study, none of the tumors (primary lesions or metastases) were positive on 18F-FLT PET/CT whereas 18F-FDG PET/CT was positive in 7 of 10 cases (24). 18F-FLT PET/CT was also found to be less sensitive than 18F-FDG PET/CT in the diagnosis of residual lymph node and distant metastases from differentiated thyroid carcinoma (25). Primary papillary thyroid carcinomas detected by 18F-FDG PET/CT were also found to have low 18F-FLT uptake, a finding that was attributed to a low proliferation activity (26).
To our best knowledge, 18F-FLT PET/CT has not been evaluated in other endocrine malignancies. Our results are consistent with the low proportion of PHEOs/PGLs with high Ki-67 indices (27–29) and provide in vivo evidence of low proliferation rates of PHEO/PGL metastases. These results are also consistent with preclinical models showing that PHEO/PGL tumor cells were mostly at the resting state of the cell cycle (the so-called G0/G1 transition) (30). 18F-FLT SUVmax was also not associated with the presence of certain criteria for poor prognosis. Resistance, invasiveness, and establishment of micrometastases of these pseudohypoxic cells might rather contribute to tumor aggressiveness than proliferation and the final outcome of a patient’s disease.
Our study also provides the first in vivo demonstration that proliferation is not a major determinant of 18F-FDG uptake in these tumors, including SDHx-related tumors, which often exhibit highly elevated uptake values (13,17). This finding has been attributed to activation of the HIF-α pathway despite normal or even high oxygen supply (also called the pseudohypoxic phenotype). On the basis of our recent metabolomic study, it has been proposed that glucose might be directly or indirectly involved in the metabolism of myoinositol/ascorbate, glutamine/glutamate, methionine/taurine, and catecholamines (31).
The main limitation of the study was related to the small number of patients evaluated by 18F-FLT PET/CT with widely heterogeneous characteristics. Some patients had also been treated in the past with therapeutic approaches that may have modified 18F-FLT uptake (Table 1). However, all of these patients were refractory to these therapies.
There is currently no effective treatment for metastatic PHEOs and PGLs. Treatment experience with cytotoxic chemotherapy using different drugs and regimens is limited and associated with high-grade toxicities (32). The most effective chemotherapy regimen appears to be the CVD scheme. A deficiency in current chemotherapy-using drugs that target dividing tumor cells has been attributed to the slow growing pattern of most PHEOs and PGLs, even metastatic ones (33). Preclinical studies have also stated that these drugs might be effective if we push experimental cells into other phases (30). Our in vivo findings also suggest that the development of antiproliferative agents may not be effective in the treatment of these tumors because primary and metastatic lesions exhibit low 18F-FLT avidity.
CONCLUSION
In this limited pilot study, 18F-FLT uptake was relatively low in PGL tumors, even metastatic and rapidly growing ones; therefore, 18F-FLT PET/CT should not be used for PGL grading and in the evaluation of their treatment responses. From a pathophysiologic standpoint, this study also provides in vivo evidence of a low proliferative rate despite high 18F-FDG uptake present in the same lesions. Nevertheless, the conclusions of the present study should be tested on a larger population of patients, including all currently known hereditary PGLs as well as recurrent and locally aggressive ones.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. No other potential conflict of interest relevant to this article was reported.
Footnotes
↵* Contributed equally to this work.
Published online Sep. 10, 2015.
- © 2015 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication April 9, 2015.
- Accepted for publication June 23, 2015.