Abstract
This prospective study compared 177Lu-ethylene diamine tetramethylene phosphonate (EDTMP) with 153Sm-EDTMP for painful skeletal metastases. Methods: Half of the 32 patients were treated with 177Lu-EDTMP and half with 153Sm-EDTMP, at 37 MBq/kg of body weight. Analgesic, pain, and quality-of-life scores (EORTC, Karnofsky, ECOG) and bone proliferation marker were used to examine efficacy. Hematologic toxicity was evaluated using NCI-CTCAE and compared between groups at baseline and each month till 3 mo after therapy. Pain relief was categorized as complete, partial, minimal, or none. Results: Pain relief with 177Lu-EDTMP was 80%: 50% complete, 41.67% partial, and 8.33% minimal. Pain relief with 153Sm-EDTMP was 75%: 33.33% complete, 58.33% partial, and 8.33% minimal. The difference was not significant (P = 1.000). Quality of life at 3 mo after therapy improved significantly in both groups as per ECOG score (P = 0.014 and 0.005 for 177Lu-EDTMP and 153Sm-EDTMP, respectively), Karnofsky index (P = 0.007 and 0.023 for 177Lu-EDTMP and 153Sm-EDTMP, respectively), and EORTC score (P = 0.004 and < 0.001 for 177Lu-EDTMP and 153Sm-EDTMP, respectively). Bone proliferation marker in responders of both groups dropped significantly (P = 0.008 for 177Lu-EDTMP and P = 0.019 for 153Sm-EDTMP), parallel to clinical response. For 177Lu-EDTMP, anemia, leukopenia, and thrombocytopenia were nonserious (grade I/II) in 46.67%, 46.67%, and 20%, respectively, and serious (grade III/IV) in 20%, 6.67%, and 0%, respectively. For 153Sm-EDTMP, anemia, leukopenia, and thrombocytopenia were nonserious (grade I/II) in 62.5%, 31.25%, and 18.75%, respectively, and serious (grade III/IV) in 18.75%, 0%, and 6.25%, respectively. One patient treated with 153Sm-EDTMP had grade IV thrombocytopenia but required no blood transfusion. Differences between groups were not significant for either nonserious or serious toxicity. For 177Lu-EDTMP, 3 of 12 responders experienced the flare phenomenon on the third day after therapy and one on the fifth day, showing no response to therapy. For 153Sm-EDTMP, 2 of 12 responders experienced the flare phenomenon, both on the third day after therapy. Conclusion: 177Lu-EDTMP has pain response efficacy similar to that of 153Sm-EDTMP and is a feasible and safe alternative, especially in centers with no nearby access to 153Sm-EDTMP.
With the increasing incidence of cancer (one of the contributory factors being increase in life expectancy due to better health care services), every year there is a rising demand to manage various advanced malignancies and their complications. Skeletal metastasis is a frequent accompaniment of end-stage malignancy. Bone metastases are most frequent in patients with advanced prostate or breast carcinoma (60%–80% of patients). Such metastases can cause debilitating pain and limit daily activities in these patients, with a significant burden to health care management and costs to society. The most typical locations are vertebrae, ribs, pelvic bones, and skull (1–3). The therapeutic options are rarely (if ever) curative, and at some point most patients with osseous metastasis develop progressive disease, leading to a series of disease-related events that have the most significant impact on quality of life (4).
The symptomatic treatment of skeletal pain due to metastases is complex and may require administration of drugs, including bisphosphonates and analgesics, and use of external-beam radiotherapy (5–7). Of the various options available, analgesics are the first step in the cascade. In cases of nociceptive pain, acetaminophen and other nonsteroidal antiinflammatory drugs are the first-line agents. They usually provide pain relief in the initial phase of disease, but as progression occurs, an increased dose or opioid analgesics are required either orally or intravenously. The use of high doses, particularly with opioid analgesics, is associated with severe adverse side effects such as nausea, constipation, confusion, drowsiness, or dry mouth. Similarly, neuropathic pain is first treated as nociceptive pain, but if there is no relief the treatment can be supplemented by tricyclic antidepressants and antiepileptic medications. Hormonal therapy and chemotherapy may be effective in alleviating the pain, but eventually the disease becomes refractory to these agents also (4). In limited metastatic disease, a short course of external-beam radiotherapy at a high dose per fraction effectively controls pain and has low toxicity, but toxicity rapidly increases as the field of irradiation increases (7,8).
In patients with multiple skeletal lesions and osteoblastic lesions on skeletal scintigraphy, systemic radiotherapy with radionuclides linked to a bone-seeking agent is an effective treatment option because of its efficacy, low cost, and comparatively low toxicity (7,9). Various radionuclides commercially available for systemic metabolic radiotherapy of bone pain include 32P, 89Sr, and 186Re chelated with hydroxyethylidene diphosphonate and 153Sm chelated with ethylene diamine tetramethylene phosphonate (EDTMP) (4,10–12). Radionuclides with high-energy β-radiation have a higher tissue range and hence high antitumor potential, but there is a proportionate increase in bone marrow suppression—a major constraint against widespread use of 32P (mean β-energy, 695 keV; half-life, 14.3 d) and 89Sr (mean β-energy, 583 keV; half-life 50.5 d), with others being long half-life (89Sr) and absence of γ-photons for imaging (4).
153Sm-EDTMP (mean β-energy, 233 keV; half-life, 1.9 d; γ-energy, 103 keV), with its short β-range emission, has advantages over radionuclides with high β-energy in terms of reduced incidence of bone marrow suppression. 177Lu chelated with EDTMP is a new and relatively inexpensive bone-seeking radiopharmaceutical and can be potentially useful for systemic therapy in patients with bone metastases (13–15). 177Lu-EDTMP (maximum β-energy, 497 keV; half-life, 6.7 d, γ-energy, 208 keV), with its short β-range emission and half-life of 6.7 d, may be a useful alternative to 153Sm-EDTMP for systemic radionuclide therapy with logistical advantages due to the relatively longer half-life of 177Lu; furthermore, 177Lu labeling with other ligands such as DOTATATE (for neuroendocrine tumors) and prostate-specific membrane antigen (for prostatic carcinoma) make it more attractive for an active therapeutic nuclear medicine program. This prospective study investigated the efficacy and safety of 177Lu-EDTMP in patients with painful bone metastases caused by tumors and compared these parameters with those of 153Sm-EDTMP.
MATERIALS AND METHODS
Inclusion and Exclusion Criteria
The patients had to meet the following criteria: bone pain due to skeletal metastases from any malignancy and bone scan demonstrating multiple osteoblastic lesions corresponding to the sites of pain. We applied standard absolute and relative exclusion criteria advocated in the guidelines (16), such as pregnancy, breastfeeding, hemoglobin level less than 9 g%, total white cell count less than 3,500/mm3, platelet count less than 100,000/mm3, acute or chronic spinal cord compression, neurogenic pain, pathologic fractures, and life expectancy less than 4 wk. Also, patients requiring surgery were excluded.
Patient Evaluation Before Inclusion
The protocol for this 18-mo prospective study was approved by the Institutional Medical Ethics Committee. All eligible patients were told about the study procedure in detail, including both the benefits and the possible adverse effects of the therapy, as well as radiation precautions and follow-up requirements after therapy. All underwent a routine workup as part of our pretherapy investigations, including clinical examination, bone scanning, and blood tests. Relevant study data from the detailed study proforma was entered into an Excel (Microsoft) spreadsheet and then analyzed.
Study Procedure
Half the patients were treated with 177Lu-EDTMP and the other half with 153Sm-EDTMP. To avoid selection bias, patients were allocated to 2 different groups using random table generation, with odd-numbered patients being given one agent and even-numbered patients the other. To be considered for 177Lu-EDTMP or 153Sm-EDTMP therapy, the patients had to have documented sites of bone metastasis causing pain severe enough to limit normal activities (based on an objective scale: a visual analog scale [VAS] or the Karnofsky index) or to require regular analgesics. The patients underwent recent (within 4 wk or less) bone scintigraphy documenting increased osteoblastic activity at the painful sites.
The patients gave written informed consent to participate in the study before being administered the radionuclide. The Atomic Energy Regulatory Board in India stipulates an activity limit of 1.1 GBq (30 mCi) of 131I at the time the patient is discharged. In an adult patient, retention of 1.1 GBq of 131I in the body corresponds to an exposure rate of about 50–60 Sv (5–6 mR)/h at a distance of 1 m. Using this parameter, the pain palliation therapy with either of the radiopharmaceuticals can be undertaken on outpatient basis.
Pain level was evaluated using a VAS; monthly for 3 mo after therapy, the patients were asked to score their pain on a scale of 0–10, where 0 indicates no pain and 10 indicates the worst pain possible. Similarly, both before therapy and monthly for 3 mo after therapy, patient performance parameters were evaluated using the Eastern Cooperative Oncology Group (ECOG) score and the Karnofsky index. Each patient’s pretherapy analgesic score was computed using the product of analgesic type and administration frequency (Table 1). The posttherapy analgesic score was evaluated at 3 mo using the same scale. The patients were asked to note when they started feeling pain relief and whether they experienced the flare phenomenon (increase in pain intensity especially during the initial few days after therapy).
A standardized therapy protocol and posttreatment procedures were maintained for both radiopharmaceuticals. Abnormalities on bone scintigraphy were correlated with an appropriate examination to exclude other causes of chronic pain unlikely to respond to treatment with bone-seeking radiopharmaceuticals. Neurogenic pain and pathologic fracture were specifically excluded. Absence of use of wide-field (hemibody) radiotherapy within 3 mo of 177Lu-EDTMP and 153Sm-EDTMP was ensured. Long-acting myelosuppressive chemotherapy (e.g., nitrosoureas) was discontinued at least 4 wk before administration of 177Lu-EDTMP and 153Sm-EDTMP and was withheld for 6–12 wk after therapy to avoid concomitant myelosuppression. A full hematologic and biochemical profile was obtained within 7 d of the proposed treatment. Disseminated intravascular coagulation—a definitive risk factor for severe thrombocytopenia after therapy—was excluded by performing pretreatment clotting studies to identify patients with subclinical disseminated intravascular coagulation. An interval of at least 48 h was ensured between bisphosphonate administration and 177Lu-EDTMP or 153Sm-EDTMP treatment.
In both groups, the dose was 37 MBq/kg of body weight (177Lu-EDTMP, 1,295–2,701 MBq; 153Sm-EDTMP, 1,258–2,553 MBq). The results for 177Lu-EDTMP and 153Sm-EDTMP at 3 mo with regard to pain relief, side effects, analgesic reduction, tumor marker reduction (e.g., bone fraction of alkaline phosphatase), and bone-scan lesion reduction were analyzed and compared. Every 15 d for 3 mo after therapy, the patients underwent a complete blood count to evaluate hematologic toxicity, and hematologic toxicity was evaluated using NCI-CTCAE, version 4.0. Both at baseline and 3 mo after therapy, the patients completed EORTC (European Organisation for Research and Treatment of Cancer) quality-of-life questionnaire BM22 to evaluate pain parameters.
Assessment of Pain Response
To enable a global assessment of posttherapy pain response, we used a predefined sliding scale that considered changes in both VAS and analgesic score (rather than a single parameter).
In a study by Yuan et al. (15) evaluating the efficacy and safety of 177Lu-EDTMP in palliation of pain from metastatic bone, breast, and hormone-refractory prostate cancer, the response to therapy was categorized as complete (disappearance of all bone pain, freely mobile, and at least a 50% reduction in the use of pain medication), partial (some improvement in bone pain), or none (no change in pain or mobility). In a study by Baczyk et al. (3) comparing the efficacy of 89Sr- and 153Sm-EDTMP in treating bone metastases from prostate and breast cancer, a complete analgesic effect was defined as a decrease in the VAS score to below 2, a partial effect as a decrease to 2–4, and an unsatisfactory response as no decrease in a VAS score below 5. In study by Tripathi et al. (4) examining 153Sm-EDTMP for palliation of pain from skeletal metastases, a responder had a decrease in VAS score by at least 2 steps for 2 wk while the analgesic score remained at least constant, a complete responder had complete relief of pain, and a nonresponder had an increase in pain score or analgesic score.
In view of this logic, the present study design considered posttherapy changes in both VAS and analgesic scores on a sliding scale. The response was categorized as complete (either pain score of 0 at 3 mo or >75% decrease in analgesic score with change in pain score), partial (either change in pain score by >3 or 50%–75% decrease in analgesic score with change in pain score), minimal (either change in pain score by 1–3 or 25%–50% decrease in analgesic score with change in pain score), or none (no change in pain score or <25% decrease in analgesic score).
RESULTS
Thirty-two patients were included in the study (26 men [81.25%] and 6 women [18.75%]; age range, 33–84 y; mean, 58.38 y). The histopathology of the primary tumor indicated that 17 patients had prostate adenocarcinoma (53.12%), 5 had breast carcinoma (15.62%), 3 had medullary carcinoma of the thyroid (9.37%), 4 had non–small cell lung carcinoma (12.5%), and 1 each had neuroendocrine tumor, renal cell carcinoma, and metastatic adenocarcinoma with unknown primary (3.12% each). In the 177Lu-EDTMP and 153Sm-EDTMP groups, the respective distributions were 6 and 11 for prostate adenocarcinoma (37.5% and 68.75%), 4 and 1 for breast carcinoma (25% and 6.25%), 1 and 3 for non–small cell lung carcinoma (6.25% and 18.75%), 3 and 0 for medullary carcinoma of the thyroid (18.75% and 0%), 1 and 0 for neuroendocrine tumor and renal cell carcinoma (6.25% and 0%), and 0 and 1 for metastatic adenocarcinoma with unknown primary (0% and 6.25%).
Table 2 presents the mean pretherapy performance characteristics of the 2 agents in the patient population.
Mean Pretherapy Performance
Hematologic toxicity was evaluated using the score on NCI-CTCAE, version 4.0. In both groups, a transient reduction in blood counts was noted after therapy, with a nadir occurring between 4 and 6 wk and gradual recovery by 8 wk. In patients treated with 177Lu-EDTMP, anemia, leukopenia, and thrombocytopenia were nonserious (grade I/II) in 46.67%, 46.67% and 20%, respectively, and serious (grade III/IV) in 20%, 6.67%, and 0%, respectively (Table 3). In patients treated with 153Sm-EDTMP, anemia, leukopenia, and thrombocytopenia were nonserious (grade I/II) in 62.5%, 31.25%, and 18.75%, respectively, and serious (grade III/IV) in 18.75%, 0%, and 6.25%, respectively. One patient treated with 153Sm-EDTMP showed grade IV thrombocytopenia; however, no blood transfusion was required (Table 3). The differences between the 2 groups were not statistically significant for either nonserious toxicity (anemia [P = 0.571], leukopenia [P = 0.511], or thrombocytopenia [P = 0.561]) or serious toxicity (anemia [P = 0.671], leukopenia [P = 0.334], or thrombocytopenia [P = 0.739]).
Hematologic Toxicity
Bone Alkaline Phosphatase (BAP) Level
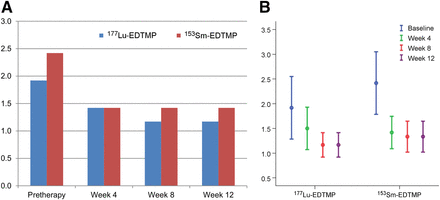
For 177Lu-EDTMP, there was a significant difference between the mean (±SD) pretherapy (34.83 ± 31.21) and posttherapy (29.04 ± 24.42) BAP levels (P = 0.008), with most responders (83.33%) showing a decrease (Fig. 1) and only 16.6% showing an increase. Likewise, for 153Sm-EDTMP, there was a significant difference between the mean pretherapy (54.49 ± 32.82) and posttherapy (46.57 ± 25.46) BAP levels (P = 0.019), with most responders (92.31%) showing a decrease (Fig. 1) and only 7.69% showing an increase.
(A) Graph demonstrating BAP levels in responders treated with 153Sm-EDTMP and 177Lu-EDTMP. (B) Error bar graph demonstrating BAP levels in responders.
Pain Score Assessment
At 3 mo after therapy, responders to 177Lu-EDTMP showed a significant reduction in mean pain score as assessed by a visual analog pain scale (3.75 ± 1.82) compared with baseline (8.25 ± 0.75) (Fig. 2) (P = 0.002). The same was true for responders to 153Sm-EDTMP (4.5 ± 1.78 at 3 mo vs. 7.67 ± 0.78 at baseline) (P = 0.002).
(A) Mean VAS score in responders treated with 153Sm-EDTMP and 177Lu-EDTMP. (B) Mean VAS score in responders treated with 153Sm-EDTMP and 177Lu-EDTMP.
Quality-of-Life Score Assessment
Quality of life (pain-free survival) in 177Lu-EDTMP responders improved significantly after therapy (mean EORTC score, 52.42 ± 12.52 before therapy vs. 42.92 ± 11.74 after therapy) (P = 0.004) (Fig. 3). The same was true for 153Sm-EDTMP responders (54.49 ± 32.82 vs. 47.57 ± 11.42) (P < 0.001).
(A) Mean EORTC index in responders treated with 153Sm-EDTMP and 177Lu-EDTMP. (B) Error bar graph illustrating mean EORTC index in responders treated with 153Sm-EDTMP and 177Lu-EDTMP.
The mean ECOG score in patients treated with 177Lu-EDTMP improved significantly between baseline (1.92 ± 1.0) and 3 mo after therapy (1.17 ± 0.39) (P = 0.014) (Fig. 4). The same was true for patients treated with 153Sm-EDTMP (2.42 ± 1.0 vs. 1.42 ± 0.51) (P = 0.005).
(A) Mean ECOG score in responders treated with 153Sm-EDTMP and 177Lu-EDTMP. (B) Error bar graph illustrating mean ECOG score in responders treated with 153Sm-EDTMP and 177Lu-EDTMP.
The mean Karnofsky index in patients treated with 177Lu-EDTMP improved significantly between baseline (65.83 ± 16.21) and 3 mo after therapy (80 ± 8.53) (P = 0.007) (Fig. 5). The same was true for patients treated with 153Sm-EDTMP (62.17 ± 16.24 vs. 75.83 ± 9.96) (P = 0.023).
(A) Graph illustrating mean Karnofsky index in responders treated with 153Sm-EDTMP and 177Lu-EDTMP. (B) Error bar graph illustrating mean Karnofsky index in responders treated with 153Sm-EDTMP and 177Lu-EDTMP.
After 177Lu-EDTMP therapy, 12 patients had pain relief (Fig. 6), 3 did not, and 1 was lost to follow-up. Relief started 3–18 d after therapy, was initially mild, gradually increased to a plateau at around 6–8 wk, and lasted about 3–13 mo. Of the 12 patients, 6 had complete relief, 5 partial, and 1 minimal. All patients with prostate cancer (osteoblastic metastases) had pain relief.
99mTc-MDP bone scan (A) and posttherapy 177Lu-EDTMP scan (B) of 69-y-old man with known case of prostate adenocarcinoma (Gleason score, 9; operated primary tumor) who presented with multiple painful skeletal metastases. Patient was on oral nonsteroidal antiinflammatory drugs 3 times per day (analgesic score, 3) and had received pelvic radiotherapy 6 mo previously. Parameters before 177Lu-EDTMP therapy: VAS, 9; BAP, 109.6; EORTC, 68; Karnofsky, 80; ECOG, 2. Posttherapy parameters: VAS, 4; BAP, 87; EORTC, 40; Karnofsky, 80; ECOG, 1. Posttherapy analgesic score was zero.
After 153Sm-EDTMP therapy, 12 patients had pain relief (Fig. 7) and 4 did not. Relief started 5–12 d after therapy, was initially mild, gradually increased to a plateau at around 6–8 wk, and lasted about 3–15 mo. Of the 12 patients, 4 had complete relief, 7 partial, and 1 minimal. Seven of the 11 patients with prostate cancer (osteoblastic metastases) had pain relief.
99mTc-MDP bone scan (A) and posttherapy 153Sm-EDTMP scan (B) of 59-y-old man with known case of prostate adenocarcinoma (Gleason score, 8; operated primary tumor) who presented with multiple painful skeletal metastases. Patient was on oral tramadol tablets 2 times per day (analgesic score, 6) and had received pelvic radiotherapy 9 mo previously. Parameters before 153Sm-EDTMP therapy: VAS, 8; BAP, 63.2; EORTC, 62; Karnofsky, 60; ECOG, 3. Posttherapy parameters: VAS, 3; BAP, 56; EORTC, 54; Karnofsky, 80; and ECOG, 1. Posttherapy analgesic score was zero.
The change in response parameters in nonresponders with either radiopharmaceutical is depicted in Table 4.
Results for Nonresponders
Flare Response, Pain Response, and Duration of Response to Therapy
In the 177Lu-EDTMP group, 3 of the 12 responders (25%) experienced the flare phenomenon on the third day after therapy and 1 (8.33%) on the fifth day after therapy, showing no response to therapy. In 153Sm-EDTMP group, 2 of the 12 responders (16.66%) experienced the flare phenomenon, both on the third day after therapy.
The response lasted about 3–15 mo both in patients treated with 177Lu-EDTMP and in those treated with 153Sm-EDTMP. Because 15 mo was the maximum follow-up period in this study, a lengthier follow-up is needed to determine if one agent has any advantage over the over in pain-free survival.
DISCUSSION
Systemic therapy with bone-seeking radiopharmaceuticals is advantageous for multiple sites of painful skeletal metastases refractory to conventional treatment. Treatment with systemic radionuclide agents is mainly palliative, reducing bone pain and preventing disease progression and, thus, the associated complications (3). An accumulation of radionuclide at a metastatic focus leads to irradiation of pathologic tissue, with β-rays destroying the cells in metastatic foci while only minimally affecting the surrounding normal tissue. Shrinkage of metastatic tumor decreases the mechanical stimulation of periosteal pain receptors (3,17–19). Some bone-seeking radiopharmaceuticals in current use are 32P, 89Sr-chloride, and 153Sm-EDTMP. Recently, the U.S. Food and Drug Administration approved another therapeutic agent for bone pain palliation, 223Ra-chloride, which is an α-emitting radioisotope similar to calcium ions that accumulates in bone and targets osteoblastic metastatic sites. Because of α-radiation, it has short tissue penetration (maximum, <10 μm) and delivers high energy per track length. Side effects are mild and predominantly gastrointestinal, with minimal myelosuppression. This agent has demonstrated promising results in castrate-resistant prostate cancer patients with bone metastases.
177Lu, a β-emitter radionuclide, besides being popular for peptide receptor radionuclide therapy, is considered a potential agent for bone pain palliation because of its suitable nuclear decay characteristics (half-life, 6.73 d; maximum β-energy, 497 keV, γ-energy, 113 keV [6.4%], 208 keV [11%]) and the feasibility of large-scale production with adequate specific activity using moderate-flux research reactors. The photons of 113 keV (6.4%) and 208 keV (11%) are suitable for imaging. EDTMP, one of the most widely used ligands, forms stable complexes with various radiometals and these complexes concentrate in the skeleton in proportion to osteoblastic activity and exhibit other favorable pharmacologic characteristics in biologic systems. Thus, 177Lu-EDTMP has favorable physical and biologic characteristics for the treatment of painful bone metastases (15,20–22).
177Lu-EDTMP studies on animals have shown significant skeletal uptake within 1 h after injection. There was no significant background activity 3 h after injection, suggesting rapid clearance of complex from the circulation. Skeletal activity was retained until 7 d after injection (14,23).
Our present study compared the clinical efficacy and safety of 177Lu-EDTMP with that of 153Sm-EDTMP in patients with painful skeletal metastasis from various malignancies. Our results showed significant pain relief, improved quality of life, reduced tumor marker in most patients, and no significant hematologic toxicity. With 177Lu-EDTMP, pain was relieved in 80% of patients overall, which is comparable to the 86% reported for 2 phase II clinical studies (23). The doses of 177Lu-EDTMP used in those 2 studies were 1,295 and 2,590 MBq (15,23). We dosed 177Lu-EDTMP at 37 MBq/kg of body weight to be comparable to the dose of 153Sm-EDTMP. With 153Sm-EDTMP, pain was relieved in 75% of patients overall, which is comparable to the 61%–95% reported for controlled clinical trials and other uncontrolled studies (24–29).
In a study by Yuan et al. (15), complete response in pain relief was observed in 55% and 80% of patients in groups 1 and 2, which were treated with 1,295 and 2,590 MBq of 177Lu-EDTMP, respectively. An improvement in Karnofsky index paralleling bone pain relief was observed, with a mean Karnofsky index of 58.18 (9.82) and 56 (8.94) at baseline in groups 1 and 2, respectively, increasing to 82.73 (9.05) at 6 wk in group 1 and 85 (5.77) in group 2.
In a study by Agarwal et al. (23), the overall response rate was 86%. Complete, partial, and minimal responses were seen in 6 (13%), 21 (48%), and 11 (25%) patients, respectively. A favorable response was seen in 27 patients (84%) with prostate cancer and 11 (92%) with breast cancer. Quality of life improved as reflected by an increase in the mean Karnofsky index from 56 ± 5 to 75 ± 7 (P < 0.0001).
Quality of life as assessed using the EORTC questionnaire in our study clearly showed a significant improvement after therapy in both groups (P = 0.004 and 0.024 for 177Lu-EDTMP and 153Sm-EDTMP, respectively). The pain relief was also associated with a significant improvement in Karnofsky index and ECOG score in both groups (respectively, P = 0.007 and 0.023 for Karnofsky index and P = 0.014 and 0.05 for ECOG score). In a study by Tripathi et al. (4) with 153Sm-EDTMP, Karnofsky index improved in all responders, with a mean score of 70.83 (SD, 8.74) before therapy versus 79.16 (SD, 9.06) at 16 wk after therapy
BAP level is currently regarded as one of the most sensitive indices of bone formation in patients with prostate cancer and skeletal metastases (30,31). Our study found that responders in both groups had significantly reduced BAP levels after therapy. Only 2 patients treated with 177Lu-EDTMP and 1 patient treated with 153Sm-EDTMP showed an increase in BAP level from baseline, but the BAP level was within the reference range in all 3 of these patients. Interestingly, nonresponders in both groups showed a trend for BAP level to rise. The reduction of BAP level in responders needs to be examined with respect to responders’ overall survival statistics compared with nonresponders.
The major limiting factor in therapy using bone-seeking radiopharmaceuticals is bone marrow toxicity. In a study by Yuan et al. (15), grade II hematologic toxicity occurred in 3 of 16 patients and grade III in 1 of 16. In addition, grade I, II, and III platelet toxicity occurred in 5, 3, and 1 patients, respectively, and grade I and II leukocyte toxicity occurred in 6 and 5 patients, respectively. In a study by Agarwal et al. (23), nonserious hematologic toxicity (grade I/II) occurred in 15 patients (34%) and serious toxicity (grade III/IV) in 10 (23%). Hematologic toxicity did not significantly differ between groups treated with 1,295 and 2,590 MBq of 177Lu-EDTMP.
In a study by Dolezal et al. (7) of 32 patients with bone-disseminated hormone-refractory prostate cancer and bone pain treated with 153Sm-EDTMP, mild and transient bone marrow suppression was observed as a side effect of treatment. None of the patients showed grade IV hematologic toxicity, and only 2 showed grade III (NCI-CTCAE). Most patients had grade I or II hematologic toxicity. In a study by Tripathi et al. (4) of 86 patients with painful skeletal metastases treated with 153Sm-EDTMP, 5 patients had grade I leukopenia and 3 had grade II. Three patients each had grade I and grade II thrombocytopenia. Eighteen patients had grade I anemia and 8 patients had grade II. None had grade III or IV hematologic toxicity.
Of our study patients treated with 177Lu-EDTMP, nonserious hematologic toxicity (grade I/II) was observed in 8 (53.33%) and serious toxicity (grade III/IV) in 4 (26.67%). Of our study patients treated with 153Sm-EDTMP, nonserious hematologic toxicity (grade I/II) was observed in 10 (62.5%) and serious toxicity (grade III/IV) in 3 (18.75%). No statistically significant differences in hematologic toxicity with regard to anemia, leukopenia, or thrombocytopenia occurred between the groups.
No statistically significant difference in nephrotoxicity occurred between the groups.
One shortcoming of the study was that the patients were included through the random table generation of odd and even numbers and were allocated to either group for therapy; this may have resulted in a difference in the distribution of primary malignancies, though prostate carcinoma made up the maximum fraction in both groups. The findings, however, indicate that 177Lu-EDTMP may be an attractive alternative that ought to be considered.
CONCLUSION
The present study showed 177Lu-EDTMP to have a pain response efficacy similar to that of 153Sm-EDTMP coupled with minimal side effects and improved quality of life. The similar hematologic toxicity profile and absence of renal toxicity showed that 177Lu-EDTMP is a feasible and safe alternative to 153Sm-EDTMP for treatment of painful skeletal metastases. Because of its longer half-life, 177Lu-EDTMP is especially useful in centers having no nearby access to 153Sm-EDTMP. This is an important consideration in view of the popularity of 177Lu for peptide receptor radionuclide therapy in many centers across the world.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. No potential conflict of interest relevant to this article was reported.
Footnotes
Published online Aug. 27, 2015.
- © 2015 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication February 11, 2015.
- Accepted for publication July 21, 2015.