Abstract
Noninvasive determination of amyloid-β peptide (Aβ) deposition has important significance for early diagnosis and medical intervention for Alzheimer's disease (AD). In the present study, we investigated the availability of radiolabeled DRM106 (123/125I-DRM106 [6-iodo-2-[4-(1H-3-pyrazolyl)phenyl]imidazo[1,2-a]pyridine]), a compound with sufficient affinity for the synthesis of human Aβ fibrils and satisfactory metabolic stability, as a SPECT ligand in living brains. Method: The sensitivity of 125I-DRM106 for detecting Aβ deposition was compared with that of 125I-IMPY (2-(4′-dimethylaminophenyl)-6-iodo-imidazo[1,2-a]pyridine), a well-known amyloid SPECT ligand, by ex vivo autoradiographic analyses in 18-mo-old amyloid precursor protein transgenic mice. To verify the sensitivity and quantitation of radiolabeled DRM106 for in vivo imaging, we compared the detectability of Aβ plaques with 123I-DRM106 and a well-known amyloid PET agent, 11C-labeled Pittsburgh compound B (11C-PiB), in 29-mo-old transgenic mice and age-matched nontransgenic littermates. Additionally, we compared the binding characteristics of 125I-DRM106 with those of 11C-PiB and 11C-PBB3, which selectively bind to Aβ plaques and preferentially to tau aggregates, respectively, in postmortem AD brain sections. Results: Ex vivo autoradiographic analysis showed that measurement with 125I-DRM106 has a higher sensitivity for detecting Aβ accumulation than with 125I-IMPY in transgenic mice. SPECT imaging with 123I-DRM106 also successfully detected Aβ deposition in living aged transgenic mice and showed strong correlation (R = 0.95, P < 0.01) in quantitative analysis for Aβ plaque detection by PET imaging with 11C-PiB, implying that sensitivity and quantitation of SPECT imaging with 123I-DRM106 are almost as good as 11C-PiB PET for the detectability of Aβ deposition. Further, the addition of nonradiolabeled DRM106 fully blocked the binding of 125I-DRM106 and 11C-PiB, but not 11C-PBB3, to AD brain sections, and 125I-DRM106 showed a lower binding ratio of the diffuse plaque–rich lateral temporal cortex to the dense-cored/neuritic plaque–rich hippocampal CA1 area, compared with 11C-PiB. Conclusion: All of these data demonstrated the high potential of 123I-DRM106 for amyloid imaging in preclinical and clinical application, and it might more preferentially detect dense-cored/neuritic amyloid deposition, which is expected to be closely associated with neuropathologic changes of AD.
- Alzheimer’s disease (AD)
- amyloid imaging
- amyloid precursor protein (APP) transgenic mouse
- DRM106
- single photon emission computed tomography (SPECT)
Alzheimer's disease (AD) is a progressive neurodegenerative disorder characterized by 2 pathologic hallmarks: amyloid-β peptide (Aβ) plaques and neurofibrillary tangles (NFTs). In vivo noninvasive detection of Aβ deposition is important for early diagnosis and medical intervention of AD at a prodromal stage, because fibrillary Aβ has already been accumulating in the brain for a few decades before AD onset (1). Molecular imaging with nuclear medicine technologies such as PET and SPECT is available for both preclinical and clinical use and is considered as a bridge between them. Over the past few years, the availabilities of several PET tracers for amyloid imaging have been successfully verified in AD patients. 11C-labeled Pittsburgh compound B (11C-PiB) is the most widely used PET ligand with which investigators have assessed the longitudinal, quantitative Aβ accumulation in AD model mice and patients (2–4) and verified amyloid deposition as a useful imaging biomarker for AD diagnosis, prognostic judgment for conversion from mild cognitive impairment to AD, and evaluation for antiamyloid therapies (5–7). To overcome the shortcoming of the limited half-life (∼20 min) of 11C-labeled tracers, ligands labeled with 18F (half-life, ∼110 min) have also been developed for further routine medical needs (8–11).
Although inferior to PET in terms of sensitivity and quantitative performance, SPECT imaging has advantages of operating cost and already-installed rate in medical hospitals, making it more suitable for primary screening for prodromal AD patients. For the past decade, several SPECT ligands have been developed for amyloid imaging. 123I-IMPY (2-(4′-dimethylaminophenyl)-6-iodo-imidazo[1,2-a]pyridine) is a SPECT tracer with high affinity for Aβ fibrils, and in vitro autoradiographic analysis has successfully detected amyloid deposition on brain sections from both AD models and AD patients (12–16). However, preliminary clinical data for 123I-IMPY showed a poor signal-to-noise ratio, making it difficult to distinguish between cognitively normal persons and AD patients, possibly because of insufficient affinity for Aβ fibrils, poor brain permeability, or metabolic instability (17). Recently, we developed a series of imidazopyridine compounds for amyloid SPECT imaging and obtained a promising candidate compound, termed DRM106 (6-iodo-2-[4-(1H-3-pyrazolyl)phenyl]imidazo[1,2-a]pyridine), which has higher affinity for synthetic human Aβ fibrils and metabolic stability. In vitro autoradiography with 125I-labeled DRM106 also successfully detected Aβ plaques in postmortem AD patient brains (18). In the present study, we performed in vivo imaging with this newly developed SPECT ligand in living model mice with AD-like amyloid pathology and compared it with 11C-PiB in the detectability of Aβ deposition.
MATERIALS AND METHODS
Radiosynthesis of Radioligands
The radiosynthesis of 125I-DRM106, 125I-IMPY, 11C-PiB, and 11C-PBB3 (2-((1E,3E)-4-(6-(11C-methylamino)pyridin-3-yl)buta-1,3-dienyl)benzo[d]thiazol-6-ol) was performed as described in previous publications (2,12,18,19). The radiochemical purity of 125I-DRM106, 125I-IMPY, 11C-PiB, and 11C-PBB3 was greater than 95%, and specific radioactivity was 81.4, 81.4, 93–354, and 72–204 GBq/μmol, respectively, at the end of synthesis.
123I-DRM106 was prepared by the reaction of its precursor with 123I-NaI in the presence of chloramine T (Fig. 1). Briefly, chloramine T (0.035 mg/20 μL 2-propanol) was added to 4.5 mL of 35 mM phosphate buffer (pH 6.3) containing 123I-NaI (29.9 GBq; Fujifilm RI Pharma Co., Ltd. [specific activity was adjusted to 714 GBq/μmol by addition of nonradiolabeled NaI]) and precursor (0.638 mg) and incubated for 5 min at room temperature, followed by quenching by the addition of 200 μL of 1N NaOH. Then the reaction mixture was incubated at 70°C for 30 min and terminated by cooling. The subsequent experimental procedure was the same, with the preparation of 125I-DRM106 as described in our previous publication (18). The radiolabeling efficiency of 123I-DRM106 was 65%–80% based on radio–thin-layer chromatography measurement. The radiochemical purity was greater than 95% at the end of synthesis, and the theoretic value of the specific activity was 714 GBq/μmol, considering that the reaction of 123I-NaI and precursor led to the formation of 123I-DRM106 with a 1:1 stoichiometric ratio.
Radiosynthesis for 123I-DRM106.
Experimental Animals
A transgenic mouse line (Tg2576), which overexpresses a mutant form of amyloid precursor protein (APPK670/671 L), was purchased from Taconic Farms Inc. Then we generated a JU-Tg2576 mouse by backcrossing of Tg2576 with a JU strain (JU/Ct-C, A.) mouse over 29 generations under license agreement of the Mayo Foundation for Medical Education and Research from Daiichi Sankyo Co. Ltd. for easier daily handling. Transgenic mice (termed JU-Tg2576 if without special description) and body weight–matched nontransgenic JU strain mice as control animals were used in the present study, except for in vivo SPECT imaging, for which commercially available Tg2576 mouse brain was used.
Preparation of Brain Homogenates and Aβ Fibrils, In Vitro Binding Assay and Aβ Assessment, In Vitro and Ex Vivo Autoradiographic Analysis and Metabolite Analysis, and Small-Animal PET and SPECT Imaging
Experimental procedures are presented in the supplemental data (supplemental materials are available at http://jnm.snmjournals.org).
Statistical Analysis
All statistical examinations in the present study were performed by SPSS software (SPSS Inc.). Statistical analyses for group comparisons were performed by Student t test or ANOVA followed by Bonferroni post hoc test. The correlation between 2 parameters was examined by parametric test with Pearson product-moment correlation coefficient (R). The difference between groups was considered significant at a P value less than 0.05. All data were expressed as mean ± SD.
RESULTS
In Vitro Binding of DRM106
Saturation curves and Scatchard analyses using a 2-site binding model demonstrated that the dissociation constant (Kd) values of high-affinity binding sites of DRM106 to synthetic human Aβ(1–40) and Aβ(1–42) fibrils, brain homogenates of transgenic mice, and AD patients were approximately 1–10 nM, and the Kd values of low-affinity sites were approximately 100–300 nM (Supplemental Fig. 1; Table 1). To estimate the correlation between quantitative 125I-DRM106 binding and Aβ amounts, brain homogenates of transgenic mice at different ages (8.1–17 mo) were used for the measurement of 125I-DRM106 binding and Aβ amounts. Age-dependent increases in 125I-DRM106 binding and Aβ amounts were detected, and there was excellent correlation between 125I-DRM106 binding and the amount of either Aβ(1–40) (R = 1, P < 0.01) or Aβ(1–42) (R = 0.99, P < 0.01) (Fig. 2).
Binding Parameters of 125I-DRM106
Correlation between amounts of Aβ species and binding of 125I-DRM106. 125I-DRM106 binding and amounts of Aβ were increased age-dependently in brain homogenates of transgenic mice at different ages as indicated, and correlations between 125I-DRM106 binding and Aβ(1–40) (A) and Aβ(1–42) (B) amounts were statistically significant. Data were from average of triplicate experiments for each age group.
Metabolite Analysis in Transgenic Mice
Solutions of 125I-DRM106 (1.1 MBq) were injected into male JU-Tg2576 transgenic mice (age, 18 mo) via the tail vein, and metabolite analyses were performed as described in our previous publication (18). Similar to results in normal rats (18), there were no detectable metabolites in the brain over the observation period, whereas only a little metabolite-related radioactivity was observable in the plasma samples, in addition to unchanged 125I-DRM106 (Fig. 3).
Metabolic stability of 125I-DRM106 in transgenic mice. Plasma and brain samples were collected at indicated time points after intravenous injection of 125I-DRM106. Thin-layer chromatography analysis clearly demonstrated that no overt metabolite in brain (lower) and only a little metabolite in plasma (upper) were detectable at the initial phase during observation period.
Ex Vivo Autoradiography with 125I-DRM106 and 125I-IMPY
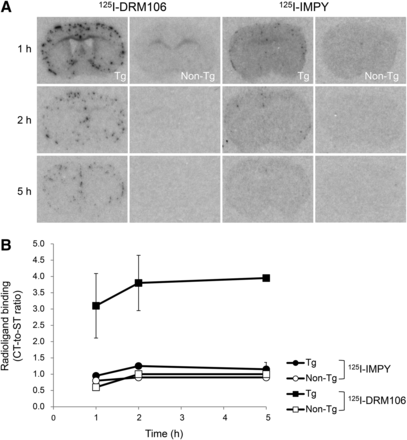
The sensitivity of 125I-DRM106 for detecting Aβ plaques was higher than that of 125I-IMPY, based on the experimental observation that more amyloid plaques were detectable using 125I-DRM106 than 125I-IMPY at 3 indicated time points in 18-mo-old transgenic mice (Fig. 4). Meanwhile, no overt difference in amyloid deposition labeled by congo red was detectable between 2 experimental groups for 125I-DRM106 and 125I-IMPY analysis (data not shown). Although slight nonspecific binding of 125I-DRM106 to white matter was detectable in both transgenic and nontransgenic mice at 1 h after injection, background radioactivity decreased to an undetectable level after 2 h (Fig. 4A), suggesting that the optimum scan time for in vivo imaging with radiolabeled DRM106 was between 1 and 2 h for acquiring good contrast with minimum loss in radioactive signals. Ex vivo emulsion autoradiography and consequent fluorescent labeling with thioflavin-S showed great consistency of 125I-DRM106 accumulation and thioflavin-S–positive Aβ deposition (Supplemental Fig. 2).
Ex autoradiographic analysis of 125I-DRM106 and 125I-IMPY in transgenic mouse brain. (A) Representative coronal images of 18-mo-old male transgenic (from left, first and third columns) and body weight–matched nontransgenic (from left, second and fourth columns) mice administered bolus injection of radiolabeled 125I-DRM106 (from left, first and second columns) and 125I-IMPY (from left, third and fourth columns) at 1, 2, and 5 h after injection. (B) Amyloidosis-associated accumulations of radioligands were quantified as ratios of radioactivity of neocortex to striatum. Data were from experiments shown in A. Transgenic (closed symbols, n = 2 for each time point) and nontransgenic (open symbols, n = 1 for each time point) mice were administered 125I-DRM106 (squares) and 125I-IMPY (circles). Data are mean ± SD. CT = neocortex; Non-Tg = nontransgenic; ST = striatum; Tg = transgenic.
In Vivo Imaging with 123I-DRM106 SPECT and 11C-PiB PET
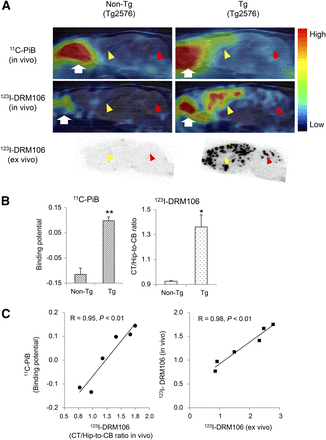
To investigate the capacity of 123I-DRM106 for in vivo detection of amyloid deposition, we performed in vivo imaging with 123I-DRM106 and 11C-PiB in the same mice and compared their quantitative analysis. The accumulation of 11C-PiB in the frontal/parietal cortex and hippocampus regions enriched with amyloid deposition was more abundant than that in other brain regions in transgenic mice (Tg2576), whereas no regional difference in radioactivity accumulation was detectable in age-matched nontransgenic mice. In vivo images of 123I-DRM106 showed great similarity to those of 11C-PiB except for more intelligible accumulation in the cerebellum (Fig. 5A; Supplemental Fig. 3). Subsequent ex vivo autoradiography also clearly demonstrated consistency in radioligand accumulation with the observation of in vivo imaging. Quantitative analysis showed that both 11C-PiB and 123I-DRM106 accumulations in amyloid pathology–enriched regions (cortex/hippocampus) in transgenic mice were significantly higher than in nontransgenic mice (Fig. 5B). Positive correlations between amyloid depositions detected by these 2 radioligands (R = 0.95, P < 0.01) and between in vivo and ex vivo binding of 123I-DRM106 (R = 0.98, P < 0.01) were statistically significant (Fig. 5C). Although using cerebellum with slight to moderate amyloid pathology as reference tissue would underestimate the binding potential (BP) calculated from the simplified reference tissue model, this would not affect our correlation analysis.
In vivo imaging with 123I-DRM106 and 11C-PiB. (A) Typical in vivo images of 11C-PiB (top) and 123I-DRM106 (middle) and ex vivo images of 123I-DRM106 (bottom) in 28-mo-old female transgenic (Tg2576; right) and age-matched nontransgenic littermate (left) mouse brains. In vivo images were overlaid on MR imaging template. Red and yellow arrowheads indicate cerebellum and cortex/hippocampus regions, respectively. White arrows indicate harderian gland. (B) Quantitative analysis for in vivo binding of 11C-PiB and 123I-DRM106 in nontransgenic (n = 3) and transgenic (n = 5) mice. In vivo bindings of 11C-PiB and 123I-DRM106 were estimated by BP and cortex-to-cerebellum ratio, respectively. Data are mean ± SD. *P < 0.05 and **P < 0.01 vs. nontransgenic mouse, t test. (C) Correlation of in vivo binding between 11C-PiB and 123I-DRM106 (left) and between in vivo and ex vivo binding of 123I-DRM106 (right). Data of mice that underwent both 11C-PiB and 123I-DRM106 scans were used for correlation analysis. Non-Tg = nontransgenic; Tg = transgenic.
Binding of 125I-DRM106, 11C-PiB, and 11C-PBB3 in Postmortem Human Brain
To evaluate binding sites of 125I-DRM106 in AD brain, we compared the in vitro autoradiographic images of 125I-DRM106 with 11C-PiB or 11C-PBB3, a radiolabeled ligand that binds to both amyloid and tau lesions at 5 nM of incubation concentration (19), in AD brain sections containing the hippocampus and lateral temporal cortex (LTCx) regions. The addition of nonradiolabeled DRM106 fully blocked the binding of 125I-DRM106 and 11C-PiB, and partially 11C-PBB3, in AD brain sections (Fig. 6A). 125I-DRM106, 11C-PiB, and 11C-PBB3 showed detectable specific binding in LTCx regions harboring numerous plaques including dense-cored/neuritic and diffuse plaques and NFTs, and in the hippocampal CA1 sector enriched with NFTs and Aβ deposition composed of numerous dense-cored/neuritic and few diffuse plaques, to greater or lesser degrees (Figs. 6B and 6C; Supplemental Fig. 4). The LTCx-to-CA1 ratio of 125I-DRM106 binding was similar to that of 11C-PBB3 but significantly lower than that of 11C-PiB (Fig. 6D).
In vitro autoradiographic analyses with 125I-DRM106, 11C-PiB, and 11C-PBB3 in postmortem AD brains. (A) Autoradiographic images of 125I-DRM106 (left), 11C-PBB3 (middle), and 11C-PiB (right) and in absence (upper) or presence (lower) of nonradiolabeled DRM106 in brain sections from patient with AD. Slices contain hippocampus, parahippocampal gyrus, and fusiform gyrus. Areas outlined with white and red squares contain hippocampal CA1 and LTCx flanking collateral sulcus, respectively, and high-power images of these 2 regions are shown in Supplemental Fig. 4. Red arrowheads indicate putative NFT-associated binding of 11C-PBB3. (B and C) Fluorescent counterstaining of NFTs (red arrows) and dense-cored/neuritic (yellow arrowheads) and diffuse (red arrowheads) plaques with FSB ((E,E)-1-fluoro-2,5-bis-(3-hydroxycarbonyl-4-hydroxy)styrylbenzene) in CA1 (B) and LTCx (C) regions. (D) Serial brain sections (5, 4, and 4 randomized sections for analyses of 125I-DRM106, 11C-PiB, and 11C-PBB3, respectively) from patient with AD were used for quantitative analyses of specific bindings of these radiolabeled ligands. There was significant main effect (F(2, 10) = 6.32, P < 0.05 by 1-way ANOVA). Post hoc analysis revealed that LTCx-to-CA1 ratio of 11C-PiB was significantly higher than those of 125I-DRM106 and 11C-PBB3 (*P < 0.05, Bonferroni). Data are mean ± SD. Scale bars: A, 5 mm; B, C, 50 μm.
DISCUSSION
For development of a SPECT ligand for amyloid imaging, the compound IMPY is a good guide because it is the first SPECT imaging agent to be tested in human subjects, and a great deal of data have already been published for reference. In vitro autography with 123I-IMPY clearly demonstrated its availability for visualization of Aβ plaques in either AD model (APP/PS1 double-transgenic mouse) or postmortem brain sections. Ex vivo autographic analysis also visually confirmed 123I-IMPY–labeled Aβ plaques in aged APP/PS1 double-transgenic mice (12). The results of in vivo imaging with micro-SPECT, however, were not encouraging (17). Subsequent clinical studies also showed that the signal-to-noise ratio for plaque labeling was not as high as that of 11C-PiB (17), indicating that successful development will require higher criteria as exemplified by higher metabolic stability and affinity for Aβ plaques than IMPY. The present and previous data clearly demonstrated that 125I-DRM106 has satisfactory metabolic stability in rodents and higher affinity for synthetic human Aβ fibrils than IMPY (18). There was high-level specific binding of 123I-DRM106 to Aβ deposition in Tg2576 mouse and postmortem AD patient brains with high affinity (Kd of 1-digit nM). This characteristic was similar to that of 11C-PiB, as published in a previous study, at low nanomolar concentrations typically used in SPECT studies (20). Such improvements in the properties of imaging agents are responsible for the superiority of 125I-DRM106 in the detectability of Aβ plaques, compared with 125I-IMPY, as shown in ex vivo autoradiographic analysis (Fig. 4). Recently, amyloid deposition in 28-mo-old Tg2576 mice was successfully visualized with a radioiodine-labeled pyridyl benzofuran derivative (21). There was, however, relatively high retention, approximately 40% of initial brain uptake, in the normal brain even after 60 min and resulting high-level nonspecific binding to white matter (21). Given that white matter in human subjects is much more abundant than in rodents, such high-level nonspecific binding to white matter may overtly affect the detectability of Aβ plaques in human subjects. Additionally, this radioligand was not metabolically stable in normal mice. The intact form in plasma was decreased to approximately 20% at 30 min after injection (21), raising the undesirable possibility that these radioactive metabolites penetrated into the brain and lowered the signal-to-noise ratio. In contrast, the amount of 125I-DRM106 remaining in the normal brain was lowered to less than 4% of initial uptake after 60 min, exhibiting excellent off-target washout (18). As a result, there was low nonspecific binding in white matter and other brain regions without amyloid pathology (Figs. 4 and 5). Additionally, a recent poster presentation—at the Alzheimer Association International Conference (22), during which a newly developed radioiodinated tracer, 123I-ABC577, was reported—demonstrated the possible availability of image-based diagnosis for AD in human subjects. Although this is a preliminary result and there is still a lack of accurate information available, such as the chemical structure of ABC577, this successful case has proved the feasibility of this SPECT agent for amyloid imaging in human subjects.
In comparison with PET, radioisotopes used in SPECT, such as 123I (half-life, 13.22 h), have a longer half-life and can therefore achieve longer distance delivery and cheaper operating cost, and more SPECT scanners have also been installed for routine clinical examinations, making it more suitable for primary screening for prodromal AD patients, especially in developing countries with large territories. Given that the sensitivity and quantitative ability of SPECT are inferior to those of PET, we need more in vivo imaging data to support the availability of DRM106 for further clinical application. We performed in vivo imaging with 123I-DRM106 in Tg2576 mice and compared this with PET imaging with 11C-PiB. Tg2576 is a widely used transgenic mouse line with AD-like amyloid pathology, with Aβ plaque density increasing exponentially from 12 mo, reaching levels similar to those seen in the AD brain (23). There were, however, many fewer amyloid-associated binding sites for 11C-PiB in the Tg2576 mouse than in AD patient tissues (2,24). Despite the fewer binding sites for 11C-PiB in Tg2576 mice, we successfully detected significant amyloid-associated 11C-PiB accumulation in the living brains, taking advantage of the high-quality 11C-PiB with high specific radioactivity up to approximately 300 GBq/umol, 6–10 times higher than the usually used level, and elderly mice (29 mo). Our comparative analysis showed great consistency in the quantitative detection of Aβ deposition between 123I-DRM106 and 11C-PiB, demonstrating that SPECT imaging with 123I-DRM106 has quantitative ability and sensitivity similar to those of PET with 11C-PiB in the living brain.
On the basis of our experimental result that the LTCx-to-CA1 ratio of binding of 125I-DRM106 is significantly lower than that of 11C-PiB, different pathologic aggregates are expected to provide major binding sites for 125I-DRM106 and 11C-PiB. Although the LTCx-to-CA1 ratio of binding of 125I-DRM106 was similar to that of 11C-PBB3, which binds to both Aβ and tau aggregates at the incubation concentration used (19), NFTs might not provide binding sites for 125I-DRM106, because a high concentration of nonradiolabeled DRM106 failed to fully block the binding of 11C-PBB3 when we postulated that NFTs provide similar binding sites for all of the β-sheet ligands. Thus, a reasonable explanation is that 125I-DRM106 might preferentially bind to dense-cored/neuritic plaques as did other amyloid tracers such as 18F-(fluorinated amyloid imaging compound of Tohoku University) (25). Given that 11C-PiB also binds to diffuse plaques, in addition to dense-cored/neuritic plaques, the lower LTCx-to-CA1 ratio of binding of 125I-DRM106 than 11C-PiB is consistent with the distribution of Aβ plaques—that is, the major Aβ deposition is dense-cored/neuritic plaques in CA1. In contrast, in addition to dense-cored/neuritic plaques, numerous diffuse plaques are also observed in the LTCx region.
The correlation between PiB binding and the amounts of either Aβ(1–40) or Aβ(1–42) was not significant (20), being attributable to preferential binding of PiB to certain Aβ subtypes, such as AβN3(pE) (2). In contrast, 125I-DRM106 binding showed excellent linearity with the amounts of either Aβ(1–40) or Aβ(1–42) in Tg2576 brain homogenates, suggesting that the terminal modification of Aβ may not change its binding sites for 125I-DRM106. If so, binding sites for 125I-DRM106 in AD patients and experimental animal models are more similar to each other, compared with that for 11C-PiB. As evidence, the ratio of maximum binding values of 125I-DRM106 for high-affinity binding sites in a 20-mo-old Tg2576 mouse to that in AD brain was proximately 0.17, much higher than that of 11C-PiB (ratio of maximum binding values in a 15-mo-old APP/PS1 double-transgenic mouse to AD brain was below 0.001) (20). On the basis of the BP values for high- and low-affinity binding sites in Tg2576 mouse brain being approximately 27 and 14, respectively, the percentage of BP for high-affinity binding is approximately 65%. Likewise, the percentage of BP for high-affinity binding in AD brain is approximately 69% (Table 1). These 2 similar values may imply a similarity of composition of binding sites for 125I-DRM106 in Tg2576 and AD brains, largely differing from that seen in 11C-PiB binding to the AD model mouse and patient brains (percentage of BP for high-affinity binding was ∼37% and 92% in the AD model mouse and patient brains, respectively) (20). This similarity in values suggests that 123/125I-DRM106 is more suitable than 11C-PiB for translational research of the progression of amyloid pathology when using existing APP transgenic mouse models, most of which express numerous dense-cored, but not AD-like, diffuse plaques.
CONCLUSION
In this study, we have successfully captured Aβ deposition in a living AD model mouse with a newly developed SPECT agent, 123I-DRM106. Given that its capacity was not inferior to 11C-PiB for detecting Aβ deposition, 123I-DRM106 has a high potential for further clinical application and, in fact, might preferentially capture the deposition of dense-cored/neuritic plaques.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. This work was supported in part by grants-in-aid for Japan Advanced Molecular Imaging Program and Core Research for Evolutional Science and Technology and Scientific Research on Innovative Areas (“Brain Environment”) 23111009 from the Ministry of Education, Culture, Sports, Science and Technology, Japan. No other potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank John Q. Trojanowski and Virginia M.-Y. Lee (Center for Neurodegenerative Disease Research, University of Pennsylvania) for kindly providing human tissue.
Footnotes
Published online Dec. 4, 2014.
- © 2015 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication August 11, 2014.
- Accepted for publication November 3, 2014.