Abstract
Accumulating evidence suggests that neurotensin receptors (NTRs) play key roles in cancer growth and survival. In this study, we developed a simple and efficient method to radiolabel neurotensin peptide with 18F for NTR-targeted imaging. Methods: The thiol-reactive reagent 18F-(2-(2-(2-fluoroethoxy)ethoxy)ethylsulfonyl)ethane (18F-DEG-VS) was facilely prepared through 1-step radiofluorination. After high-pressure liquid chromatography purification, 18F-DEG-VS was incubated with the c(RGDyC) and c(RGDyK) peptide mixture to evaluate its specificity toward the reactive thiol. Thiolated neurotensin peptide was then labeled with 18F using this novel synthon, and the resulting imaging probe was subjected to receptor-binding assay and small-animal PET studies in a murine xenograft model. The imaging results and metabolic stability of 18F-DEG-VS-NT were compared with the thiol-specific maleimide derivative N-[2-(4-18F-fluorobenzamido)ethyl]maleimide-neurotensin (18F-FBEM-NT). Results: 18F-DEG-VS was obtained in high labeling yield. The reaction of 19F-DEG-VS was highly specific for thiols at neutral pH, whereas the lysine of c(RGDyK) reacted at a pH greater than 8.5. 18F-DEG-VS-c(RGDyC) was the preferred product when both c(RGDyK) and c(RGDyC) were incubated together with 18F-DEG-VS. Thiolated neurotensin peptide (Cys-NT) efficiently reacted with 18F-DEG-VS, with a 95% labeling yield (decay-corrected). The radiochemical purity of the 18F-DEG-VS-NT was greater than 98%, and the specific activity was about 19.2 ± 4.3 TBq/mmol. Noninvasive small-animal PET demonstrated that 18F-DEG-VS-NT had an NTR-specific tumor uptake in subcutaneous HT-29 xenografts. The tumor-to-muscle, tumor-to-liver, and tumor-to-kidney ratios reached 30.65 ± 22.31, 11.86 ± 1.98, and 1.91 ± 0.43 at 2 h after injection, respectively, based on the biodistribution study. Receptor specificity was demonstrated by blocking experiment. Compared with 18F-FBEM-NT, 18F-DEG-VS-NT was synthesized with fewer steps and provided significantly improved imaging quality in vivo. Conclusion: We have established a facile 18F-labeling method for site-specific labeling of the Cys-NT. Using this method, we synthesized an NTR-targeted PET agent, which demonstrated high tumor-to-background contrast.
PET imaging with 18F has several attributes that make it clinically attractive, including nearly 100% positron efficiency, very high specific radioactivity, and a short half-life of 18F (109.8 min) (1). Moreover, its low positron energy and attendant short positron linear range in tissue result in higher resolution in PET imaging than other more energetic positron emitters. On the other hand, the half-life of 18F is sufficient to allow syntheses, transportation, and imaging procedures to be extended over several hours, while the patient is subjected to a limited amount of radiation exposure. Recently, 18F-labeled biomolecules, including peptides with short biologic half-lives, are gaining increasing importance for PET imaging studies of receptor expression and activity in living subjects. However, direct labeling of peptides with 18F typically results in poor incorporation yields, and the harsh conditions (such as high temperature, high pH, anhydrous conditions, and organic solvents) may lead to degradation of the peptide ligands. Therefore, peptides are typically labeled with 18F by an indirect strategy that involves incorporating 18F into a prosthetic group first, followed by conjugation to the peptide precursor under mild conditions (2–8).
Because free thiol groups are not as common as amino and carboxylic acid in most peptides and proteins, thiol-reactive prosthetic groups have been used to modify peptides and proteins at specific sites, providing an 18F-labeling approach with high chemoselectivity in contrast to the carboxylate and amine-reactive reagents (9–12).
Despite some encouraging results, most thiol-reactive synthons require multistep reactions that are time-consuming and labor-intensive. Thus, there is a clear need to develop a more facile procedure for 18F labeling of thiols. Vinyl sulfone (VS) holds several advantages for 18F labeling of thiol groups, compared with 18F-labeled maleimide. First, the product from the reaction of thiol with VS yields a single stereoisomer structure, unlike maleimide conjugates, which have 2 potential stereoisomers (13,14). Second, 18F-VS synthon might be obtained through a 1-step 18F-fluorination, greatly simplifying the synthetic procedure. And third, VS conjugates are stable in aqueous solution for extended periods and are not subject to hydrolysis at neutral pH like maleimide conjugates (15–19). Thus, VS has an excellent potential for conjugation to thiol-containing peptides or other biomolecules even when used in aqueous buffer conditions.
In this study, we report the development of a novel 18F-labeling agent, 18F-(2-(2-(2-fluoroethoxy)ethoxy)ethylsulfonyl)ethane (18F-DEG-VS), for conjugation to free thiol groups in bioligands. Because accumulating evidence suggests that neurotensin receptors (NTRs) play key roles in cancer growth and survival (20,21), a thiolated neurotensin (NT) peptide was used to test the potential of 18F-DEG-VS as a peptide-labeling agent. The resulting 18F-labeled-NT derivative was further evaluated by PET in a rodent model with an NTR1-positive tumor.
MATERIALS AND METHODS
General
All commercially available chemical reagents were purchased from Aldrich and used without further purification. c(RGDyC) and c(RGDyK) were purchased from Peptides International Inc. A thiolated NT peptide analog was synthesized by the City of Hope peptide synthesis core using standard FMOC chemistry. No-carrier-added 18F-fluoride was produced via the 18O(p, n)18F reaction. All high-performance liquid chromatography (HPLC) conditions are gradient. HPLC methods, nuclear magnetic resonance spectra, and mass spectrometry details are listed in the supplemental material (available at http://jnm.snmjournals.org).
Chemistry
Detailed synthetic procedures and characterizations were provided as supplemental material for 2-(2-hydroxyethoxy)ethyl 4-nitrobenzenesulfonate (1), 2-(2-(2-(vinylsulfonyl)ethoxy)ethoxy)ethyl 4-nitrobenzenesulfonate (2), (2-(2-(2-fluoroethoxy)ethoxy)ethylsulfonyl)ethene (19F-DEG-VS, 3), 19F-DEG-VS-c(RGDyC), 19F-DEG-VS-c(RGDyK), 19F-DEG-VS-NT, 19F-DEG-VS-(Ac)-NT, 19F-FBEM-c(RGDyC), and 19F-FBEM-NT.
Radiochemistry
Detailed labeling procedures and characterizations were provided as supplemental material for 18F-DEG-VS, 18F-DEG-VS-c(RGDyC), 18F-DEG-VS-c(RGDyK), 18F-DEG-VS-NT, 18F-DEG-VS-(Ac)-NT, 18F-FBEM-c(RGDyC), and N-[2-(4-18F-fluorobenzamido)ethyl]maleimide-neurotensin (18F-FBEM-NT).
Selectivity Test: c(RGDyK) and c(RGDyC)
18F-DEG-VS (100 μL, 37 MBq [1 mCi]) and 50 μL of borate buffer (pH 8.5) were added into c(RGDyC) (100 μg, 0.16 μmol) and c(RGDyK) (105 μg, 0.16 μmol). The reaction mixture was incubated at room temperature for 30 min. The reaction was quenched by acetic acid (5%, 600 μL), and the product was analyzed by radio-HPLC using Method 1 (supplemental data).
Cells and Animals
The human colon adenocarcinoma cell HT-29 was obtained from American Type Culture Collection. Animal procedures were performed according to a protocol approved by the University of Southern California Institutional Animal Care and Use Committee. In the procedure, 4- to 6-wk-old male athymic mice (BALB/c nu/nu; weight, 20–30 g) were injected subcutaneously with HT-29 human colon adenocarcinoma cells at a concentration of 1 × 106 cells per 0.1 mL in the shoulder, and enough time was allowed for tumors to grow to at least 3 mm in diameter.
In Vitro Cell-Binding Assay
The in vitro NTR1-binding affinities of 19F-DEG-VS-NT and NT(8-13) were assessed via competitive cell-binding assays using 125I-NT(8-13) (PerkinElmer) as described previously (supplemental material) (22).
Biodistribution and Small-Animal PET Imaging of HT-29 Tumor Xenografts in Mice
Small-animal PET imaging was performed in nude mice bearing HT-29 colorectal xenografts after tail vein injection with 3.7 MBq of 18F-DEG-VS-NT or 18F-FBEM-NT (n = 3, respectively). For blocking studies, NT(8-13) (100 μg) was coinjected with 18F-DEG-VS-NT (n = 3). Serial imaging (0.5, 1, and 2 h after injection; scan duration, 5, 5, and 10 min, respectively) was performed using a small-animal PET R4 scanner (Siemens Medical Solutions, Inc.).
Biodistributions were performed in nude mice bearing HT-29 colorectal xenografts. Animals were sacrificed under inhalation anesthesia at 2 h after injection of 3.7 MBq of 18F-DEG-VS-NT. Tissues and organs of interest were excised and weighed. Radioactivity in each excised specimen was measured using a γ counter; radioactivity uptake was expressed as percentage injected dose per gram (%ID/g). The mean uptake (%ID/g) and corresponding SD was calculated for each group of animals.
In Vivo Metabolic Stability
The in vivo metabolic stability of 18F-DEG-VS-NT and 18F-FBEM-NT was evaluated in nude mice bearing HT-29 tumors (supplemental material). For 18F-DEG-VS-c(RGDyC) and 18F-FBEM-c(RGDyC), the urine samples were collected and analyzed by HPLC.
Statistical Analysis
Quantitative data were expressed as mean ± SD. Means were compared using 1-way ANOVA and the Student t test. P values of less than 0.05 were considered statistically significant.
RESULTS
Chemistry
As shown in Figure 1, the F-DEG-VS precursor [2-(2-(2-(vinylsulfonyl)ethoxy)ethoxy) ethyl 4-nitrobenzenesulfonate 2 was synthesized in 20% yield after a 2-step reaction and was 19F-fluorinated by reacting with tetrabutylammonium fluoride (TBAF). 19F-DEG-VS was used as a chemical standard for 18F-DEG-VS and the starting material for the following reactions.
(A) Synthetic scheme of F-DEG-VS. (B) HPLC profile of crude reaction of 18F-DEG-VS. (C) HPLC profile of 18F-DEG-VS at 4 h after purification.
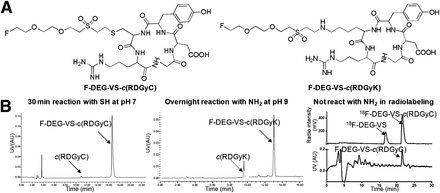
We first evaluated the cold reaction between 19F-DEG-VS and c(RGDyC) (with a free SH group) and c(RGDyK) (with a free NH2 group) peptides (Fig. 2A). Both c(RGDyC) and c(RGDyK) reacted with 19F-DEG-VS; however, c(RGDyK) reacted only at a pH higher than 8.5 after overnight incubation, compared with a pH of 7.0 and 30-min incubation for c(RGDyC) (Fig. 2B).
(A) Chemical structure of F-DEG-VS-c(RGDyC) and F-DEG-VS-c(RGDyK). (B) HPLC profile of crude reaction of 19F-DEG-VS-c(RGDyC), 19F-DEG-VS-c(RGDyK), 18F-DEG-VS with c(RGDyC)/c(RGDyK) (radio), and standard of 19F-DEG-VS-c(RGDyC) on radio-HPLC (UV). AU = arbitrary units; UV = ultraviolet.
Thiolated NT also reacted with 19F-DEG-VS efficiently. However, 2 products (19F-DEG-VS-NT and (19F-DEG-VS)2-NT) (Figs. 3A and 3B) were obtained when the reaction was performed at a high pH (e.g., pH 8.5), due to the reaction of F-DEG-VS with 1 thiol and 1 amine group present in our NT analog.
(A) Radiosynthesis scheme of 18F-DEG-VS-NT. (B) HPLC profile of crude reaction of 19F-DEG-VS and NT. (C) HPLC profiles of 18F-DEG-VS-NT (radioactive) 19F-DEG-VS-NT (UV), and (19F-DEG-VS)2-NT (UV). AU = arbitrary units; UV = ultraviolet.
Radiochemistry
The 18F labeling of the VS synthon was tested at various solvent, concentration, and temperature conditions. With 10-mg precursor loading, the representative radio-HPLC trace of the crude labeling reaction is shown in Figure 1B. Although the radiolabeling yield for 18F-DEG-VS was calculated to be 90% yield based on HPLC integration, the isolation yield was 35% ± 6%. The discrepancy between these 2 calculation methods could be caused by the activity that was bound to reaction vessel walls and the head of HPLC columns. 18F-DEG-VS demonstrated good stability, and the radiochemical purity was still more than 99% at 4 h after HPLC purification after incubation in phosphate-buffered saline (Fig. 2C).
For the radiolabeling reaction, we incubated equal molar amounts of c(RGDyC) and c(RGDyK) with 18F-DEG-VS in the same reactor. In this case, we observed only the product from c(RGDyC) and 18F-DEG-VS (Fig. 2B). The absence of product from c(RGDyK)/18F-DEG-VS demonstrated the selectivity of this reaction for the thiol over the amino group. Thus, even at pH 8.5, 18F-DEG-VS selectively labeled the thiol group in the presence of a free amino group.
18F-DEG-VS also efficiently reacted with thiolated NT. The (F-DEG-VS)2-NT byproduct seen with the 19F synthesis was not observed in radiolabeling reactions as shown by HLPC (Fig. 3C). The radiochemical purity of 18F-DEG-VS-NT was still greater than 95% at 5 h after HPLC purification after incubation in phosphate-buffered saline (Supplemental Fig. 1). To compare 18F-DEG-VS with a thiol-specific maleimide-based reagent, 18F-FBEM was also prepared and shown to react efficiently with c(RGDyC) and thiolated NT, respectively (Supplemental Fig. 2).
In Vitro Cell-Binding Affinity
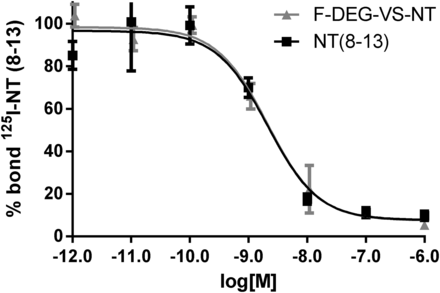
We compared the receptor-binding affinity of 19F-DEG-VS-NT with that of NT(8-13) using a competitive cell-binding assay (Fig. 4). Both peptides inhibited the binding of 125I-NT(8-13) to NTR1-positive HT-29 cells in a dose-dependent manner. The IC50 (the half maximal inhibitory concentration) value for 19F-DEG-VS-NT (2.03 ± 0.22 nmol/L) was comparable to that of NT(8-13) (2.12 ± 0.26 nmol/L). The results clearly demonstrated that F-DEG-VS-NT has an in vitro receptor-binding affinity to NTR1 similar to NT(8-13).
Competitive binding assays of 125I-NT(8-13) and 19F-DEG-VS-NT or NT(8-13) in HT-29 cells. Data are mean ± SD (n = 3). x-axis reflects concentration of nonradiolabeled competitor. IC50 values for 19F-DEG-VS-NT and NT(8-13) were 2.03 ± 0.22 and 2.12 ± 0.26 nmol/L, respectively.
Biodistribution and Small-Animal PET Imaging
The NTR1-targeting efficacy of 18F-DEG-VS-NT was evaluated in HT-29 xenografts (NTR1-positive) at multiple time points (0.5, 1, and 2 h after injection) with small-animal PET. As shown in Figure 5A, the HT-29 tumors were clearly visualized with high tumor-to-background contrast for 18F-DEG-VS-NT, and the tumor uptake was 1.30 ± 0.17, 0.96 ± 0.29, and 0.63 ± 0.20 %ID/g at 0.5, 1, and 2 h after injection, respectively. In comparison, 18F-FBEM-NT also demonstrated prominent uptake in tumors (0.13 ± 0.08 %ID/g, 2 h after injection) but was significantly (P = 0.017) lower than observed for 18F-DEG-VS-NT (2 h after injection). The liver and kidney uptake of 18F-FBEM-NT was 0.28 ± 0.03 and 0.64 ± 0.02 %ID/g at 2 h after injection, respectively. Compared with 18F-FBEM-NT, 18F-DEG-VS-NT exhibited superior tumor-to-background contrast and lower abdomen background (Supplemental Fig. 3).
(A) Representative coronal small-animal PET images of mice bearing HT-29 xenografts after injection of 3.7 MBq of 18F-DEG-VS-NT without (upper) and with unradiolabeled NT(8-13) (middle) and 3.7 MBq of 18F-FBEM-NT (lower). (B) Biodistribution of 18F-DEG-VS-NT in mice bearing HT-29 xenograft at 2 h after injection (p.i.).
The NTR1 specificity of 18F-DEG-VS-NT was confirmed by a blocking experiment in which the radiotracer was coinjected with an excess of unlabeled NT(8-13). As can be seen from Figure 5A, in the presence of unlabeled NT(8-13), the NTR1 tumor uptake (0.47, 0.15, and 0.04 %ID/g at 0.5, 1, and 2 h after injection, respectively) was significantly (P < 0.01) lower than that without NT(8-13) blocking at all time points. The kidney uptake rapidly decreased from 6.82 ± 2.90 %ID/g at 0.5 h after injection to 0.17 ± 0.01 %ID/g at 2 h after injection. On the basis of imaging analysis, tumor-to-kidney, tumor-to-liver, and tumor-to-muscle ratios were 3.69 ± 1.50, 10.33 ± 4.01, and 27.12 ± 5.46 at 2 h after injection, respectively. At 2 h after injection, significantly higher uptake was demonstrated in the tumors than in the kidney, liver, and muscle. Other organs, including heart and lung, were essentially at background levels by 2 h after injection.
In addition to the small-animal PET study, we also performed biodistribution studies using a separate group of HT-29 tumor–bearing mice at 2 h after injection of 18F-DEG-VS-NT. As shown in Figure 5B, the tumor and kidney uptake was 0.86 ± 0.09 and 0.46 ± 0.05 %ID/g, respectively. At 2 h after injection, significantly higher uptake was demonstrated in the tumors than in the kidney, liver, and muscle (Fig. 5B). Other organs, including the heart and lung, were essentially at background levels by 2 h after injection.
Metabolic Stability Study
The metabolic stability of 18F-DEG-VS-NT was determined in mouse urine and in the liver, kidneys, and HT-29 tumor homogenates at 1 h after injection. The high-performance liquid chromatograms are shown in Supplemental Figure 4. The retention time of intact 18F-DEG-VS-NT was 18.50 min. A major metabolite peak was found at about 20 min for the tumor, and 2 major metabolite peaks were found at 13–15 and 17 min for urine, kidney, and liver samples. No significant defluorination was observed throughout the study. The metabolic stability of 18F-FBEM-NT was also studied. The retention time of intact 18F-FBEM-NT was 19.02 min. Besides the multiple peaks between 15 and 21 min, there was a major peak at about 5 min for tumor, kidney, and liver. There was no substantial peak for unmetabolized material in the tumor at 1 h after injection. We also synthesized the 18F-DEG-VS-c(RGDyC) and 18F-FBEM-c(RGDyC) and analyzed their urine metabolites. Unlike the unstable NT peptide, the c(RGDyC) is stable in vivo. Accordingly, 18F-DEG-VS-c(RGDyC) gave a single peak corresponding to intact labeled peptide excreted in urine (Supplemental Fig. 5). 18F-FBEM-c(RGDyC) also gave a major peak, with evidence of small amounts of other components (Supplemental Fig. 6).
DISCUSSION
Accumulating evidence suggests that NTRs play key roles in cancer growth and survival (20,23,24). In fact, NTRs have been proposed as a promising marker for human pancreatic carcinoma (25), breast cancer (26), head and neck carcinoma (27), prostate cancer (28), and non–small cell lung cancer (29).
The development of imaging agents to obtain NTR expression profiles or fingerprints of individual tumors could therefore lead to efficient early-stage diagnosis and customized treatment options for cancer patients. NT, a tridecapeptide ligand for NTR, is metabolized rapidly in plasma by endogenous peptidases. To improve the in vivo stability, various NT analogs have been developed. For example, several radiolabeled NT analogs were recently developed as a valuable tool for both imaging and therapy of NTR-positive tumors (30–35). Although encouraging results have been obtained in these initial studies, the relatively high kidney uptake and suboptimal tumor-to-tissue contrast warrant further improvement of these agents, especially in the choice of the radiolabel. Previously, we have demonstrated that the pharmacokinetics of peptide-based PET probes improved significantly by substituting 18F in place of 64Cu (36,37). In fact, as one of the commonly used PET radioisotopes, 18F can also be easily produced in high quantities in a medical cyclotron, with an ideal half-life of 110 min for imaging applications. Therefore, we have devoted a significant amount of effort to develop a 18F-labeled PET probe for NTR-targeted imaging.
Because cysteines are much less abundant than lysines, aspartic acid, and glutamic acid residues in peptides and proteins, thiol-reactive agents have been used to site-selectively modify these biomolecules. Previously, several thiol-reactive 18F synthons have been reported, most of which bear a maleimide group for conjugate addition of thiols under mild conditions. However, maleimide-based synthons require multistep reactions, which are time-consuming and labor-intensive. In recognition of these issues, we set out to develop a platform technology based on the Michael addition reactivity of vinyl sulfone. VS chemistry has been demonstrated to be suitable for the selective modification of cysteine residues under mild conditions (15). The water stability of the VS function, the lack of byproducts, the almost quantitative yields of the reaction with thiols, and the chemical stability of the thioether linkage formed make this reaction an appealing approach for 18F labeling.
Our VS-based prosthetic group was designed to be hydrophilic, a highly desirable attribute for in vivo applications. To simplify the labeling procedures, initial efforts were focused on a 1-step radiofluorination of VS. Radiofluorination reactions were performed in MeCN/dimethyl sulfoxide using azeotropically dried 18F-TBAF. Conversion of VS synthon to the corresponding 18F-DEG-VS was found to be strongly dependent on the VS synthon reaction concentration and temperature. Under optimized conditions, greater than 90% labeling yield could be obtained within 15 min based on HPLC integration. The isolated yield was determined to be 35% ± 6%.
Because VS may also react with primary amines at high pH (15), we decided to explore the selectivity of the VS synthon reaction under neutral pH conditions. Although both c(RGDyK) and c(RGDyC) efficiently react with 19F-DEG-VS reagent, c(RGDyK) is much less reactive, which required overnight incubation and higher pH, compared with 30-min incubation for c(RGDyC). Moreover, with the 18F-DEG-VS reagent, only c(RGDyC) reacted in the equimolar mixture of c(RGDyK) and c(RGDyC). This result clearly demonstrated the chemoselectivity of 18F-DEG-VS toward the SH functional group.
After establishing an efficient 18F-DEG-VS–labeling method, thiolated NT peptide was labeled with 18F. 18F-DEG-VS-NT was obtained in greater than 95% yield within 35 min, and the radiochemical purity was more than 99%. The specific radioactivity of 18F-DEG-VS-NT was determined on the basis of a literature method (38) in which the ultraviolet integration of final product was compared with a standard titration curve, yielding a specific radioactivity of 19.2 ± 4.3 TBq/mmol for 18F-DEG-VS-NT.
Because the chemical modification of a peptide can significantly decrease receptor binding affinity, an in vitro cell-binding assay of 19F-DEG-VS-NT and NT(8-13) was performed. The difference of the binding affinity between 19F-DEG-VS-NT and NT(8-13) was negligible, as supported by the similar IC50 values. The imaging quality of 18F-DEG-VS-NT was evaluated in vivo using an HT-29 xenograft model, which has been well established to have high NTR1 expression (20). 18F-DEG-VS-NT had a rapid renal clearance. 18F-DEG-VS-NT predominantly accumulated in the kidneys (6.82 ± 2.90 %ID/g) at 30 min after injection and quickly decreased to 1.19 ± 0.49 %ID/g at 1 h after injection and 0.17 ± 0.01 %ID/g at 2 h after injection. In the blocking experiment, nonradioactive NT peptide significantly (P < 0.01) inhibited the tumor uptake of 18F-DEG-VS-NT (Fig. 5A) at all time points, clearly demonstrating the receptor specificity of this imaging agent. Because the tracer has low uptake in the abdominal region, we also performed a biodistribution study to determine the uptake in liver, kidney, small intestine, spleen, stomach, and other organs or tissues more accurately. As shown in Figure 5B, the uptake in these organs was close to background level, correlating well with the high-contrast tumor image obtained from the small-animal PET scan. Because the activity decreased rapidly in all major organs, a high tumor-to-organ ratio was obtained at 2 h after injection, including tumor to muscle (30.65 ± 22.31), tumor to liver (11.86 ± 1.98), and tumor to kidneys (1.91 ± 0.43), calculated by biodistribution study. Because NT peptide has 2 potential reactive sites, (Ac)-NT was additionally synthesized, which could also be efficiently labeled with 18F-DEG-VS (Supplemental Fig. 7). It still needs to be determined whether this new agent will have tumor-targeting capability similar to 18F-DEG-VS-NT.
Although the major focus of this report was the development of a new thiol-specific, 18F-radiolabeling method for peptides and its evaluation by PET imaging, it was also important to perform a direct in vivo comparison with an existing maleimide-based reagent to demonstrate the potential advantages and limitations of our newly developed labeling strategy. Therefore, a maleimide-based reagent, namely 18F-FBEM-NT, was also synthesized for a direct comparison of imaging quality and metabolic stability. As shown in Supplemental Figure 2, 18F-FBEM-NT was a more complicated synthesis involving 5 steps: fluorination, hydrolysis, activation of the carboxyl group, reaction with the maleimide derivative, and conjugation with Cys-NT. In comparison, the synthesis of 18F-DEG-VS-NT involves only 2 steps: fluorination of the synthon and conjugation to Cys-NT. Importantly on PET imaging, 18F-FBEM-NT demonstrated significantly higher background and lower contrast than 18F-DEG-VS-NT (Supplemental Fig. 3). To determine whether the lower abdominal background and high tumor uptake observed for 18F-DEG-VS-NT, compared with 18F-FBEM-NT, were caused by a difference in metabolism, a metabolic stability study was performed. As shown in Supplemental Figure 4, both 18F-DEG-VS-NT and 18F-FBEM-NT exhibited several metabolites in vivo. However, a major metabolite peak at about 5 min was found for 18F-FBEM-NT but not for 18F-DEG-VS-NT. Nonetheless, because of the short half-life of NT in humans and rodents (39,40), a conclusion cannot be drawn based on just the above experiments. In a further approach, we have conjugated the more metabolically stable c(RGDyC) peptide with both synthons and studied the urine metabolism of 18F-FBEM-c(RGDyC) and 18F-DEG-VS-c(RGDyC). As shown in Supplemental Figures 5 and 6, 18F-FBEM-c(RGDyC) has additional metabolites in urine not seen for 18F-DEG-VS-c(RGDyC). This experiment suggests that our VS-based synthon is more stable in vivo than the maleimide-based synthon. Thus, the metabolites observed in the 18F-DEG-VS-NT study may be caused mainly by the degradation of the NT peptide and not the synthon itself. Clearly, the stability benefit of the DEG-VS linker, compared with the maleimide linker, may also depend on the peptide to which it is conjugated. Although maleimide-based synthons such as 18F-FBEM are expected to be unstable based on literature reports, they may still perform well within the time scale of an 18F imaging study. The observed superior contrast of 18F-DEG-VS-NT, compared with 18F-FBEM-NT, in an animal model warrants consideration of clinical translation of this agent for NTR-targeted imaging in humans.
CONCLUSION
We have established a novel 18F-labeling method for site-specific labeling a free thiol group present or introduced into peptide or proteins. Using this novel method, we synthesized a NTR1-targeted PET imaging agent, which demonstrated specific tumor uptake and superior tumor-to-background contrast. The elevated tumor–to–major-organ uptake ratios (including tumor to kidney) led to high-contrast images at 2 h after injection. The low background in normal tissues should allow the detection of small tumors (especially at the abdomen area) by PET imaging 18F-DEG-VS-NT.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. This work was supported by a grant from Larry L. Hillblom Foundation (2013-D-015-SUP), the Jonas Bros Foundation, the Juvenile Diabetes Research Foundation (37-2011-638), the American Cancer Society (121991-MRSG-12-034-01-CCE), and the USC Department of Radiology. No other potential conflict of interest relevant to this article was reported.
Footnotes
Published online May 22, 2014.
- © 2014 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication January 15, 2014.
- Accepted for publication March 17, 2014.