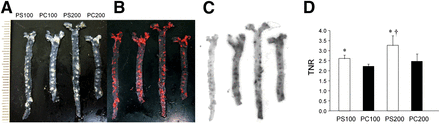Abstract
Macrophage infiltration is a common characteristic feature of atherosclerotic-vulnerable plaques. Macrophages recognize phosphatidylserine (PS) exposed on the surface of apoptotic cells, which triggers the engulfment of the apoptotic cells by macrophages through phagocytosis. In this study, we prepared radiolabeled PS liposomes for detection of vulnerable plaques. Methods: PS liposomes were prepared by lipid film hydration. Phosphatidylcholine (PC) liposomes were prepared as controls. Liposomes (100 or 200 nm) were generated by an extruder to produce PS100, PS200, PC100, and PC200 liposomes. These were then radiolabeled by encapsulating 111In-nitrilotriacetic acid using an active-loading method. 111In liposomes were incubated with cultured macrophages for 2 h, and the uptake level was measured. For biodistribution studies, the 111In liposomes were injected intravenously into ddY mice. In addition, the 111In liposomes were injected into apolipoprotein E–deficient (apoE−/−) mice, and the aortas were harvested for autoradiography and oil red O staining. For SPECT imaging, 111In liposomes were injected intravenously into Watanabe heritable hyperlipidemic rabbits and scanned 48 h after injection. Results: The radiochemical yields were greater than 95% for all the prepared 111In liposomes. The level of in vitro uptake by macrophages was 60.5, 14.7, 32.0, and 14.4 percentage injected dose per milligram of protein for 111In-PS100, 111In-PC100, 111In-PS200, and 111In-PC200, respectively. In biodistribution studies, high spleen uptake was seen with PC liposomes. Liver uptake was high for all liposomes but was lowest with 111In-PS200. The blood half-lives were 3.2, 22.0, 3.6, and 7.4 min for 111In-PS100, 111In-PC100, 111In-PS200, and 111In-PC200, respectively. The distribution of 111In-labeled PS liposomes into atherosclerotic regions determined by autoradiography was well matched with the results of oil red O staining in apoE−/− mice. The target-to-nontarget ratios were 2.62, 2.23, 3.27, and 2.51 for 111In-PS100, 111In-PC100, 111In-PS200, and 111In-PC200, respectively. The aorta was successfully visualized by SPECT at 48 h after 111In-labeled PS liposome injection; however, high liver uptake was also observed. Discussion: From the in vitro uptake study, it has been demonstrated that macrophage targeting was accomplished by PS modification. Also, an atherosclerotic region was successfully detected by 111In-PS200 in apoE−/− mice and Watanabe heritable hyperlipidemic rabbits in vivo. Liposome modification to obtain slower blood clearance and lower liver uptake would be required to improve the SPECT images.
Vulnerable plaques in atherosclerotic lesions can cause acute myocardial infarction and stroke. Therefore, detection of high-risk, vulnerable plaque is important for risk stratification and to facilitate early treatment. Macrophage infiltration is a characteristic of vulnerable plaques, and macrophages play a central role in the destabilization of atherosclerotic lesions (1,2). We and other groups have previously reported that because macrophages are metabolically active, vulnerable plaques can be detected with the glucose analog 18F-FDG by PET (3–6). Thus, 18F-FDG PET can be a useful tool for detecting vulnerable plaques, and the method has been used in both animal investigations and clinical studies (7–10). However, glucose consumption is not a specific phenomenon for macrophages, and glucose concentration affects 18F-FDG accumulation in macrophages (11,12). Thus, blood sugar level needs to be well controlled for accurate evaluation of vulnerable plaques by 18F-FDG. Because diabetes often coexists with atherosclerosis, this would create some difficulties in such patients. Furthermore, there would be some difficulties associated with coronary artery imaging, because of physiologic accumulation of 18F-FDG in the heart.
In this study, we developed a liposome-based imaging probe that targets macrophages. Liposomes have been shown to be safe for human use, and they can be a suitable platform for multimodal imaging (13–15). In addition, it is well known that macrophages phagocytize particles such as liposomes (16,17). Furthermore, we applied surface modification to the liposomes for more effective macrophage targeting. In atherosclerotic-vulnerable plaques, many apoptotic cells are produced in a sequence of inflammatory reactions. In atherosclerotic regions, macrophages recognize phosphatidylserine (PS) on the surface of apoptotic cells, which triggers the macrophages to phagocytize the apoptotic cells (18–21). Therefore, in this study, we prepared PS-modified liposomes and radiolabeled them with 111In for SPECT imaging. We first evaluated the effect of PS modification of the liposomes on macrophage uptake in vitro. After investigating the in vivo kinetics of the liposomes in normal mice, imaging studies were performed in apolipoprotein E–deficient (apoE−/−) mice for quantitative analysis, and in Watanabe heritable hyperlipidemic (WHHL) rabbits for SPECT imaging.
MATERIALS AND METHODS
Materials
Distearoylphosphatidylcholine and distearoylphosphatidyl-l-serine were purchased from NOF Corp. Diethylenetriaminepentaacetic acid and 8-hydroxyquinoline were purchased from Wako Pure Chemicals. 111In-InCl3 was obtained from Nihon Medi-Physics Co., Ltd. All other chemicals used were reagent grade. Male ddY mice were supplied by Japan SLC Co., Ltd. ApoE−/− mice were purchased from The Jackson Laboratory. WHHL rabbits and New Zealand White rabbits were purchased from Kitayama Labes Co., Ltd. The present animal study was approved by the Animal Care and Use Committee of the Hamamatsu University School of Medicine.
Synthesis of Dipalmitoylphosphatidyl d-Serine
Dipalmitoylphosphatidyl d-serine was synthesized according to the procedure of Lindh and Stawińsky (22). The details are described in the Supplemental Appendix (supplemental materials are available at http://jnm.snmjournals.org).
Preparation of Liposomes
PS-modified liposomes and control liposomes (phosphatidylcholine [PC] liposomes) were prepared. To investigate the specificity for PS, which incorporates the naturally occurring l-serine form, phosphatidyl-d-serine (D-PS)–containing liposomes (D-PS liposomes) were also prepared. Prepared sizes were 100 and 200 nm for each liposome type.
Liposomes were prepared by the lipid film hydration-extrusion method (23). The details are described in the Supplemental Appendix.
Radiolabeling of Liposomes
The liposomes were radiolabeled by the remote-loading method. Briefly, 111In-InCl3 solution was mixed with 51 mM oxine (8-quinolinol) in ethanol and incubated in 2 M sodium acetate buffer for 10 min at 40°C to prepare the loading solution. Nitrilotriacetic acid (NTA)–encapsulated liposomes were incubated in the loading solution for 10 min at 40°C for transchelation between NTA and oxine. The excess 111In-oxine was removed by ultracentrifugation (37,500g, 30 min). The mechanism of radionuclide transport through the lipid bilayer can be summarized as follows. Oxine is a lipophilic chelating ligand that can be distributed to both the aqueous phase and the liposomal lipid membrane. Under the indicated experimental conditions, the oxine concentration is approximately 1,000- to 100,000-fold in excess of 111In, meaning that practically all 111In ions are coordinated to oxine at equilibrium. The resulting 111In-oxine complex diffuses across the lipid bilayer from outside of the liposome to inside, followed by transchelation with NTA (a stronger chelator) inside the liposome aqueous core. Thus, 111In-labeled PS liposomes (100 and 200 nm [111In-PS100 and 111In-PS200, respectively]), 111In-labeled D-PS liposomes (100 and 200 nm [111In-DPS100 and 111In-DPS200, respectively]), and 111In-labeled PC liposomes (100 and 200 nm [111In-PC100 and 111In-PC200, respectively]) were prepared.
Macrophage Isolation
Mouse peritoneal macrophages were isolated as previously described, with slight modifications (24). Four days after the 10% thioglycolate injection (intraperitoneal) into female ddY mice, the animals were sacrificed and macrophages were isolated from the peritoneal cavity by phosphate-buffered saline (PBS) lavage. Macrophages were plated into 6-well plates and cultured in Dulbecco modified Eagle medium (glucose, 4,000 mg/L; Sigma-Aldrich Co.) supplemented with 10% fetal bovine serum and penicillin and streptomycin (100 U/mL) at 37°C in 95% air–5% CO2.
111In-Labled Liposome Uptake by Cultured Macrophages
Plated macrophages were cultured for 24 h and washed with PBS. Macrophages were then preincubated with 2 mL of Dulbecco modified Eagle medium at 37°C for 1 h. Subsequently, 7.4 kBq of 111In-PS100, 111In-DPS100, 111In-PC100, 111In-PS200, 111In-DPS200, or 111In-PC200 liposomes were added to wells and incubated at 37°C for 2 h in 95% air–5% CO2. In addition, for controls, 111In-InCl3 (7.4 kBq) was added to additional wells. After incubation, the medium was removed, and the cells were rinsed twice with 1 mL of PBS. The washings were combined with the removed medium. Macrophages were scraped from the wells in 0.35 mL of PBS, and the wells were rinsed with an additional 0.35 mL of PBS, which was combined with the cell suspension. The radioactivity of medium and cell suspension was measured with an automated γ counter (Wizard2 3”; Perkin Elmer Inc.). After radioactivity measurement, the protein concentration was determined by the method of Lowry et al. (25). Uptake of 111In-labeled liposomes was expressed as the ratio of radioactivity in the cell to the initial dose per milligram of protein.
Biodistribution Studies
Male ddY mice (6 wk) were injected with 37 kBq of 111In-PS100, 111In-PC100, 111In-PS200, or 111In-PC200 liposomes via the tail vein (n = 4 for each time-pint). At the designated time intervals, the mice were sacrificed and their organs were dissected. Tissues were weighed, and radioactivity was measured by automated γ counting. Data were calculated as the percentage injected dose per gram of tissue. The blood curves were fitted with PMOD software (PMOD Technologies Ltd.), and the half-lives were determined.
Ex Vivo Autoradiography in apoE−/− Mice
Twenty-four apoE−/− mice (27 wk old, n = 6) were used for ex vivo autoradiography studies. Five-week-old ApoE–/– mice were fed a high-fat diet (Clinton/Cybulsky Rodent Diet D12108 with 1.25% cholesterol; Research Diets) for 22 wk. The mice received injections of 111In-PS100, 111In-PC100, 111In-PS200, or 111In-PC200 (5.6 MBq, 100 μL) via the tail vain. Two hours after injection, the mice were euthanized by an overdose of sodium pentobarbital by injection, and the aortic arch and aorta were fixed by cardiac perfusion with 10% neutral-buffered formalin. Dissected arteries were opened longitudinally and exposed on a phosphor imaging plate (Fuji Imaging Plate BAS-UR; Fujifilm Corp.) for 19 h. Autoradiographic images were obtained and analyzed by a computerized imaging analysis system (Bio-Imaging Analyzer FLA 3000; Fujifilm Corp.). Additionally, aortic tissues were stained with oil red O for plaque area determination. Five regions of interest were placed on the plaque area (target) and the nonplaque area (nontarget) in the aortic tissue, and 3 ROIs were placed for background area around each aorta in autoradiography images. Signal intensities were shown as photostimulated luminescence per unit area (PSL/mm2), and average values for each were used for the analysis. The target-to-nontarget ratios (TNRs) were calculated as follows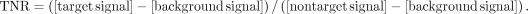
The timeline diagram is given in Figure 1A.
Timeline diagrams for animal studies. (A) Ex vivo autoradiography in apoE−/− mice. (B) SPECT imaging, ex vivo autoradiography, and histologic analysis in WHHL rabbits. ARG = autoradiography; i.v. = intravenously.
SPECT/CT Imaging with WHHL Rabbits
WHHL rabbits (n = 2 per group for 111In-PS100 and 111In-PS200; age, 17 mo; weight, 3.3–3.5 kg) or control New Zealand White rabbits (n = 2 for 111In-PS200; age, 17 mo; weight, 3.4 kg) were used for SPECT imaging studies. The animals were anesthetized, and 111In-PS100 or 111In-PS200 (74 MBq) was injected into a marginal ear vein. SPECT scanning was performed 48 h after injection of liposomes using an FX system PET/SPECT/CT scanner (Gamma-Medica Inc.). After the SPECT study, a CT angiogram was acquired. The details are described in the Supplemental Appendix.
After the last scan, rabbits were sacrificed by an overdose of sodium pentobarbital. The aorta was removed, and 10- and 5-μm-thick consecutive sections were prepared. The autoradiogram was obtained with a phosphor imaging system (FLA-3000; Fujifilm Corp.) with 10-μm-thick sections. Other 5-μm-thick sections were subjected to immunohistochemical staining for macrophages, Azan-Mallory staining, and oil red O staining. Immunohistochemistry was performed according to the method reported by Tsukada et al. using the rabbit macrophage-specific monoclonal antibody RAM-11 (Dako Corp.) (26), and the slices were costained with hematoxylin for identification of the nucleus. The timeline of the experiment is shown in Figure 1B.
Statistical Analysis
Data are presented as the mean ± SD. Statistical analysis was performed using the Mann–Whitney U test or paired t test for comparisons between or within groups, respectively. Statistical significance was established at a P value of less than 0.05.
RESULTS
In Vitro Uptake of 111In Liposomes by Macrophages
The in vitro uptake of 111In-labeled liposomes and 111In-InCl3 by mouse peritoneal macrophages is summarized in Figure 2. Uptake of both sizes of liposomes was significantly higher for PS than for PC liposomes. 111In-PS100 showed significantly higher uptake than 111In-PS200. d-serine liposomes accumulated in macrophages, but the level was lower than for l-serine liposomes of each size. Only a slight uptake of 111In-InCl3 was observed.
111In-labeled liposome uptake by cultured macrophages. Significantly higher uptake was observed in PS liposomes than PC liposomes of each size (*P < 0.05). d-isomer of PS liposomes showed lower uptake than l-isomers.
Biodistribution Studies in Normal Mice
Biodistribution data are summarized in Tables 1–4⇓⇓⇓. High liver uptake was observed for all liposomes investigated. Uptake into the spleen was higher for PC liposomes than PS liposomes. Blood clearance was faster for PS liposomes than PC liposomes, and 111In-PC100 showed the slowest clearance. The blood half-lives were 3.2, 22.0, 3.6, and 7.4 min for 111In-PS100, 111In-PC100, 111In-PS200, and 111In-PC200, respectively.
Biodistribution of 111In-PS100 in Normal Mice
Biodistribution of 111In-PS200 in Normal Mice
Biodistribution of 111In-PC100 in Normal Mice
Biodistribution of 111In-PC200 in Normal Mice
Ex vivo Autoradiography in apoE−/− Mice
En face ex vivo autoradiography showed accumulation of all investigated 111In liposomes in the plaque area in mice (Figs. 3A–3C). Radioactive regions were well matched with oil red O staining. The uptake to nonspecific regions was higher in PC liposomes, and the TNRs were lower in PC liposomes than PS liposomes (Fig. 3D). The TNRs were 2.62 ± 0.16, 2.23 ± 0.13, 3.27 ± 0.49, and 2.51 ± 0.34 for 111In-PS100, 111In-PC100, 111In-PS200, and 111In-PC200, respectively.
En face autoradiography of apoE−/− mice aorta. Photographic images of unstained aorta (A), images after oil red O staining (B), autoradiograms (C), and TNR (D). Autoradiography results were consistent with results of oil red O staining. TNR was significantly higher in PS liposomes than PC liposomes for all liposome sizes (*P < 0.05). TNR was higher for PS200 than PS100 (†P < 0.05).
SPECT Imaging, Ex Vivo Autoradiography, and Histologic Analysis in WHHL Rabbits
Figure 4 summarizes the SPECT, autoradiography, and histologic images in WHHL and normal rabbits. The arrows indicate the position of the aorta. The atherosclerotic regions were successfully visualized with 111In-PS200 and 111In-PS100, and the clearest image was obtained with 111In-PS200. No aortic accumulation was seen in normal rabbits with 111In-PS200. Autoradiography images of the aortic section showed accumulation of radioactivity in the plaque area in WHHL rabbits. High accumulation of radioactivity was observed in the macrophage foam cell area, and radioactivity was low in the fibrotic area as shown by Azan-Mallory staining.
SPECT and CT images, ex vivo autoradiography, and histologic images of 111In-PS100 (A) and 111In-PS200 (B) in WHHL rabbits and 111In-PS200 in normal rabbit (C). Arrows indicate position of aorta. L represents liver. Magnified images of Azan-Mallory staining show macrophage foam cell–rich region with less smooth muscle cells (dashed red circle) and a more fibrotic region with dead macrophages (dashed yellow circle). Atherosclerotic regions were successfully visualized with 111In-PS200 and 111In-PS100 in WHHL rabbits. Radioactivity was accumulated in macrophage foam cell area and was low in fibrotic area. No aortic accumulation was seen in normal rabbit.
DISCUSSION
Macrophage infiltration plays a pivotal role in plaque rupture by releasing inflammatory cytokines and proteases; therefore, it is reasonable to target macrophages for vulnerable plaque imaging. Also, it is well known that macrophages are predisposed to phagocytize particles such as liposomes (16,17), and several liposome-based imaging probes have been reported recently (27,28). Therefore, in this study, we used liposomes as carriers for the atherosclerotic region and designed a SPECT imaging probe. In fact, cultured macrophages took up control PC liposomes to a certain extent (Fig. 2). Uptake was higher with 100- versus 200-nm liposomes. In addition, when the eat-me signal, PS, was incorporated into the liposome, uptake into cultured macrophages was significantly elevated; furthermore, the uptake of D-PS into macrophages was lower than that of the naturally occurring l isoform of PS (Fig. 2). These results suggest that macrophage targeting using liposomes was successful and that target specificity was enhanced by PS modification. D-PS liposomes showed higher accumulation in macrophages than in PC liposomes for each liposome size. The negative charge on the surface of the liposome may enhance particle capture by macrophages. We previously reported that 18F-FDG uptake into cultured mouse peritoneal macrophages was 48.8 percentage dose per milligram of protein (%dose/mg protein) with a 3-h incubation (29). In this study, the uptake of 111In-PS100 (60.5 % dose/mg protein) was greater than that of 18F-FDG. These results show the potential of 111In-PS100 for in vivo atherosclerotic plaque imaging.
The atherosclerotic regions were successfully visualized with 111In-labeled PS liposomes in apoE−/− mice with en face autoradiography after the intravenous injection of the tracers. In contrast with the in vitro results, accumulation was higher for 111In-PS200 than 111In-PS100. Atherosclerotic lesions were visualized by 111In-labeled PC liposomes of both sizes, but nonspecific accumulation was higher with PC liposomes than PS liposomes; thus, TNRs were higher for PS liposomes than PC liposomes. The highest TNR was obtained with 111In-PS200 in the en face autoradiography in apoE−/− mice. Also, successful SPECT imaging was possible with 111In-PS200 in WHHL rabbits. Thus, 111In-PS200 showed the best features for in vivo imaging, although 111In-PS100 provided the best results in in vitro investigations.
In the biodistribution study in normal mice, 111In-PS200 showed somewhat slower blood clearance than 111In-PS100. This difference may result in higher accumulation of 111In-PS200 than 111In-PS100 in atherosclerotic regions of apoE−/− mice, although the uptake was higher in 111In-PS100 in in vitro experiments. Also, we observed a slower blood clearance of PC liposomes than PS liposomes. These results suggest that slow blood clearance should accelerate liposome accumulation into the plaque. However, in atherosclerosis imaging, signals from the blood pool disturb the visualization of plaque in the vessel wall; thus, blood clearance of the imaging probe should ideally be not too slow, and excessive liver uptake makes it difficult to visualize the small atherosclerotic region in coronary arteries. In general, PEGylation of particles prolongs the blood clearance because of the avoidance from the reticuloendothelial system, and liver accumulation would be decreased by PEGylation (30). However, because the high liver accumulation can be explained by the liposome uptake into the Kupffer cells, which have characteristics similar to macrophages concerning phagocytosis, the too-low accumulation to the liver would cause the decreased uptake to the macrophages in atherosclerotic lesions. Therefore, for atherosclerosis imaging, a certain level of PEGylation of liposomes is desirable, which prolongs blood clearance to a limited extent and which does not disturb phagocytosis by macrophages. Further investigation of liposome PEGylation would be required, to determine the optimal balance between macrophage-targeting ability and desirable blood clearance. In addition, the prolonged retention to the liver might have come from the transchelation of 111In with transferrin (31,32). Radiolabeling with 99mTc might improve the kinetics and reduce the radiation dose. Liposomes can act as a platform for various other imaging modalities, such as MR imaging and optical imaging. Recently, several liposome-based atherosclerosis imaging probes for various imaging modalities have been reported (33–35). Because liposomes are biocompatible and have long histories as drug carriers for human use (36), such imaging probes, including the liposome system we have described, should be good candidates for clinical use in the future.
CONCLUSION
In this study, the atherosclerotic region was detectable by macrophage targeting with radiolabeled liposomes (PS liposomes), although additional investigations would be needed to improve the in vivo biodistribution and plaque accumulation level.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. This work was supported by grants-in-aid for scientific research from the Japan Science and Technology Agency (JST), Japan Society for the Promotion of Science (JSPS), and Japanese Ministry of Health, Labour and Welfare. No other potential conflict of interest relevant to this article was reported.
Footnotes
Published online Dec. 12, 2013.
- © 2014 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication March 14, 2013.
- Accepted for publication September 3, 2013.