Abstract
Angiotensin II receptor blockers (ARBs) are a common treatment for hypertensive patients but affect renal function. In this study, the effects of ARB on 18F-FDG distribution and excretion were examined in mice treated with telmisartan at different doses. Methods: Male C57BL/6J mice were given telmisartan (low-dose group, 0.33 mg/kg/d; moderate-dose group, 0.66 mg/kg/d; high-dose group, 3 mg/kg/d) mixed in a high-fat diet for 20 wk. Mice on a telmisartan-free diet served as the control. At designated time points, the mice were injected with 18F-FDG (18.5 MBq/mouse, n = 5–10/time point for each group) to examine its biodistribution. Autoradiography using kidney sections was performed to visualize 18F-FDG excretion. Plasma blood urea nitrogen (BUN) and creatinine levels were also measured to evaluate renal function. Results: Twenty-week telmisartan treatment significantly and dose-dependently increased 18F-FDG levels in the blood (percentage injected dose per gram of tissue normalized by animal body weight: low, 0.13 ± 0.03 [P < 0.0083]; moderate, 0.15 ± 0.01 [P < 0.0083]; high, 0.15 ± 0.03 [P < 0.0083], vs. control, 0.09 ± 0.01). Significantly increased 18F-FDG levels in organs were observed in mice in the moderate- and high-dose groups but not in the low-dose group. The plasma BUN and creatinine levels also dose-dependently increased, but they were within the reference ranges (for BUN: low, 27.00 ± 4.42 mg/dL; moderate, 28.40 ± 2.70 mg/dL; high, 39.22 ± 6.91 mg/dL [P < 0.0083], vs. control, 22.40 ± 2.80 mg/dL. For creatinine: low, 0.28 ± 0.11 mg/dL; moderate, 0.40 ± 0.07 mg/dL [P < 0.0083]; high, 0.51 ± 0.09 mg/dL [P < 0.0083], vs. control, 0.18 ± 0.04 mg/dL). The blood 18F-FDG level positively correlated with plasma BUN (r = 0.48, P < 0.01) and creatinine (r = 0.61, P < 0.01) levels. The 18F-FDG levels in the blood and organs returned to baseline 3 wk after cessation of telmisartan treatment. Autoradiography indicated that renal 18F-FDG excretion was attenuated by telmisartan treatment and was reversed after treatment cessation. Conclusion: 18F-FDG levels in the blood and organs were significantly increased by telmisartan treatment, indicating a potential increase in background activity on PET imaging of patients treated with ARBs. Our findings indicate the need for a careful assessment of 18F-FDG uptake in patients treated with ARBs. A brief cessation of ARB treatment may be a potential method to avoid these effects and solve this problem.
Hypertension is highly prevalent in the general population. Clinical studies have demonstrated the antihypertensive efficacy of angiotensin II receptor blockers (ARBs) and angiotensin-converting enzyme inhibitors (ACEIs) (1), but these 2 types of medication also have side effects on renal function (2). The renal protective properties of ACEIs and ARBs have been mentioned by several clinical experts and investigators (3); however, the subsequent fall in glomerular filtration rate (GFR) associated with attenuation of proteinuria has also been observed in clinical trials (4,5).
PET using 18F-FDG is an established diagnostic imaging technique in oncology, cardiology, and neurology (6). As for the excretion of 18F-FDG, it is incompletely reabsorbed at proximal tubules, unlike glucose, and is excreted through the urinary system (7). Therefore, 18F-FDG excretion may be reduced by an impairment of renal function, such as a decreased GFR. Recently, a clinical study showed that patients with suspected renal failure have increased 18F-FDG levels in the blood pool together with high plasma creatinine levels (8).
Accordingly, ARBs may affect 18F-FDG excretion and distribution and consequently change 18F-FDG uptake in target tissues. Difficulties in diagnosis in clinical practice may result, because quantification of glucose metabolism by 18F-FDG PET plays an important role in nuclear medicine diagnosis (9), and daily clinical practice using 18F-FDG PET includes not only visual inspection but also quantitative analysis, such as determination of standardized uptake value (SUV) (10). On the other hand, optimization of urinary 18F-FDG excretion during PET imaging with diuretic agents has also been applied in a clinical setting (11), because reduction of radioactivity in the urinary tract is helpful to avoid interference in evaluations of the abdominopelvic region (12). ARB or ACEI may also have such a potential value if they can reversibly reduce the GFR and consequently reduce 18F-FDG excretion.
In the present study, we investigated the effects of ARBs on 18F-FDG distribution and excretion in C57BL/6J mice treated with different doses of telmisartan.
MATERIALS AND METHODS
Animal Studies
Animal care and all experimental procedures were performed with the approval of the Animal Care Committee of Hokkaido University. Studies were performed on male C57BL/6J mice obtained from CLEA Japan Inc. After the age of 6 wk, the mice were fed a high-fat diet (21% fat and 0.15% cholesterol) with or without telmisartan (T1644; LKT Laboratories, Inc.). The doses of telmisartan were set at 0.33 mg/kg/d (low dose), 0.66 mg/kg/d (moderate dose), and 3 mg/kg/d (high dose); these were calculated from the daily clinical doses of 20, 40, and 160 mg, respectively. The food intake and body weight of each animal were monitored weekly to adjust the amount of telmisartan mixed into the diet. All animals were kept in a temperature-controlled facility in the Laboratory of Animal Experiments at Hokkaido University, on a 12-h light cycle with free access to food and water.
The experimental design and treatment schedule are shown in Supplemental Figure 1 (supplemental materials are available online only at http://jnm.snmjournals.org). Telmisartan treatment was continued for a maximum of 20 wk. At the designated time points (at 1, 2, 10, and 20 wk of telmisartan treatment and 1, 2, and 3 wk after treatment cessation), the following experiments were performed: analysis of biodistribution of 18F-FDG, measurement of plasma blood urea nitrogen (BUN) and creatinine levels, and autoradiography of kidney sections. Mice on the telmisartan-free diet served as age-matched controls corresponding to the telmisartan-treated group (at 20 wk of treatment and 2 and 3 wk after treatment cessation) and were used to obtain baseline levels before the treatment started.
Biodistribution of 18F-FDG
At each experimental time point (n = 5–10/time point), mice were kept fasting for 12 h, anesthetized with pentobarbital (45 mg/kg of body weight, intraperitoneally), and injected with 18F-FDG (18.5 MBq/mouse, obtained from Hokkaido University Hospital, which produces the tracer for clinical use). Blood glucose level in all animals was measured immediately before the 18F-FDG injection. Two hours after the 18F-FDG injection, blood samples were collected under deep pentobarbital anesthesia for subsequent analyses. Organs were removed and weighed. Radioactivity in the blood and each organ was measured in a well-type scintillation counter (1480 Wizard 3″; Wallac Co., Ltd.) for 60 s, together with appropriate 18F standards and the background tubes. The decay-corrected total counting rate of a sample with an error rate of less than 5% was used and was converted to percentage injected dose per gram of tissue normalized by animal body weight ([%ID/g] × kg).
Plasma BUN and Creatinine Levels
The blood samples collected from each animal were centrifuged after the addition of 100 U of heparin to obtain the plasma. BUN and creatinine levels in plasma samples were determined using Vet-Test 8008 (IDEXX Laboratories). Standards were supplied by the manufacturer.
Autoradiographic Studies
To assess renal 18F-FDG excretion, intrarenal 18F-FDG distribution was determined by autoradiography using kidney sections. One side of the kidney of each animal was rapidly removed after sacrifice, embedded in Tissue-Tek medium (Sakura Finetechnical Co., Ltd.), and frozen in isopentane and dry ice. Cross sections of 10-μm thickness were immediately cut and thaw-mounted on glass slides. Then, the cryostat cross sections were exposed to phosphor imaging plates (BAS-SR 2025; Fuji Photo Film Co., Ltd.) for 12 h to detect the radioactivity of 18F (13). After each exposure, the imaging plates were scanned with a bioimaging analyzer (BAS-5000, 25-μm internal resolution; Fuji Photo Film Co., Ltd.) and the obtained images were analyzed with image analysis software (Multi Gauge, version 3.0; Fuji Photo Film Co., Ltd.).
Statistical Analysis
Numeric parameters were expressed as mean ± SD. Linear regression analysis was performed correlating plasma BUN or creatinine level with 18F-FDG level in the blood. A 2-tailed value of P less than 0.05 was considered statistically significant. One-way ANOVA was used to assess the significance of differences among the 4 animal groups (i.e., control, low-dose, moderate-dose, and high-dose groups). Bonferroni adjustment was implemented for post hoc comparison, and the statistical significance of differences was determined using a 2-tailed P value of less than 0.05/6 (i.e., 0.0083).
RESULTS
Body Weight, Plasma BUN Levels, Creatinine Levels, and Blood 18F-FDG Levels
Before telmisartan treatment, the mice had a body weight of 19.0 ± 1.1 g. The mice treated with high-dose telmisartan showed a significantly lower body weight than the control mice after 20 wk of treatment (low, 37.9 ± 1.0 g; moderate, 35.7 ± 2.1 g; high, 30.2 ± 2.9 g [P < 0.0083], vs. control, 37.1 ± 3.3 g) and after the 3-wk treatment cessation (low, 39.6 ± 2.5 g; moderate, 40.1 ± 1.7 g; high, 34.0 ± 2.5 g [P < 0.0083], vs. control, 40.3 ± 3.1 g).
Plasma BUN, plasma creatinine, and blood 18F-FDG levels are shown in Supplemental Figure 2. Mice in the control group did not show any significant changes in BUN, creatinine, and 18F-FDG levels during the experimental period. In the telmisartan-treated groups, increases in BUN, creatinine, and 18F-FDG levels were observed in the first 2 wk of treatment and remained at the same levels until cessation of the treatment at 20 wk (for BUN: low, 27.00 ± 4.42 mg/dL; moderate, 28.40 ± 2.70 mg/dL; high, 39.22 ± 6.91 mg/dL [P < 0.0083], vs. control, 22.40 ± 2.80 mg/dL. For creatinine: low, 0.28 ± 0.11 mg/dL; moderate, 0.40 ± 0.07 mg/dL [P < 0.0083]; high, 0.51 ± 0.09 mg/dL [P < 0.0083], vs. control, 0.18 ± 0.04 mg/dL. For 18F-FDG: low, 0.13 ± 0.03 [%ID/g] × kg [P < 0.0083]; moderate, 0.15 ± 0.01 [%ID/g] × kg [P < 0.0083]; high, 0.15 ± 0.03 [%ID/g] × kg [P < 0.0083], vs. control, 0.09 ± 0.01 [%ID/g] × kg).
After cessation of the treatment, all parameters decreased to baseline levels (for BUN: low, 23.80 ± 2.59 mg/dL; moderate, 25.40 ± 2.30 mg/dL; high, 30.00 ± 3.54 mg/dL [P < 0.0083], vs. control, 22.60 ± 2.88 mg/dL. For creatinine: low, 0.18 ± 0.04 mg/dL; moderate, 0.20 ± 0.00 mg/dL; high, 0.20 ± 0.00 mg/dL, vs. control, 0.22 ± 0.11 mg/dL. For 18F-FDG: low, 0.09 ± 0.01 [%ID/g] × kg; moderate, 0.10 ± 0.00 [%ID/g] × kg; high, 0.11 ± 0.01 [%ID/g] × kg, vs. control, 0.10 ± 0.02 [%ID/g] × kg).
Correlations between 18F-FDG and BUN levels and between 18F-FDG and creatinine levels were also examined (Fig. 1). 18F-FDG levels correlated positively with BUN (r = 0.48, P < 0.01) and creatinine levels (r = 0.61, P < 0.01).
Graphs showing positive correlations between 18F-FDG and BUN levels (r = 0.48, P < 0.01) (A) and between 18F-FDG and creatinine levels (r = 0.61, P < 0.01) (B).
Biodistribution of 18F-FDG
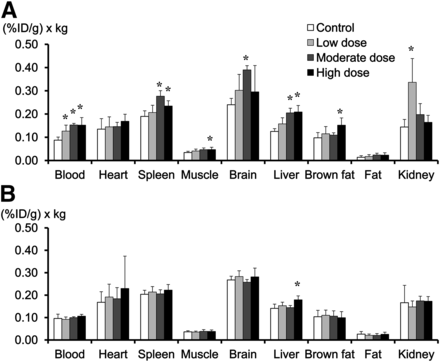
Figure 2 shows the 18F-FDG distribution at 20 wk of telmisartan treatment and after treatment cessation. In mice given 20 wk of moderate- or high-dose treatment, 18F-FDG levels in the blood and several organs were significantly higher than those in the control group (Fig. 2A). Three weeks after treatment cessation, 18F-FDG levels in the blood and organs decreased to baseline levels (Fig. 2B).
(A) In mice given 20 wk of telmisartan treatment, 18F-FDG accumulation levels in blood, muscle, liver, and spleen were significantly higher in moderate-dose group and high-dose group than in control group. (B) After 3-wk treatment cessation, 18F-FDG levels in blood and organs were same as those in control group. *P < 0.0083 for Bonferroni post hoc test vs. control group.
Autoradiographic Images of 18F-FDG in Kidney
Figure 3 shows autoradiographic images of intrarenal 18F-FDG distribution. In mice before the start of telmisartan treatment, 18F-FDG accumulated mainly in the inner part of the medulla. After telmisartan treatment, the intensive accumulation of 18F-FDG in that location markedly decreased to be as low as that in the cortex. Three weeks after treatment cessation, an intensive accumulation of 18F-FDG was again observed in the inner part of the medulla.
Autoradiographic images of 18F-FDG in kidney sections. Red arrows indicate medulla, and blue arrows indicate cortex. In mice before start of telmisartan treatment (*1), 18F-FDG markedly accumulated in inner part of medulla. During telmisartan treatment, intensive accumulation of 18F-FDG in inner part of medulla markedly decreased to be as low as that in cortex. Two or 3 wk after treatment cessation (*2), gradual recovery of 18F-FDG accumulation was observed at inner part of medulla. w = weeks.
DISCUSSION
In this study, we showed that telmisartan treatment dose-dependently increased plasma BUN and creatinine levels in mice and that the 18F-FDG level in the blood concomitantly increased (Supplemental Fig. 2). 18F-FDG level positively correlated with BUN and creatinine levels (Fig. 1). 18F-FDG levels in several organs were significantly higher in mice treated with moderate- and high-dose telmisartan (Fig. 2). Renal 18F-FDG excretion was attenuated by the telmisartan treatment (Fig. 3). Moreover, the results indicated that these parameters returned to baseline levels 3 wk after treatment cessation.
With the above-mentioned findings, we demonstrated that ARB treatment affected renal function and 18F-FDG uptake in the blood and organs of mice. In clinical settings, quantification of glucose metabolism by 18F-FDG PET is an established diagnostic technique. The levels of 18F-FDG uptake in target tissues provide important information for evaluating the malignancy of tumors and the viability of the myocardium and for identifying the location of seizure foci. In particular, as a semiquantitative parameter, SUV is widely used to evaluate tumor malignancy. Moreover, in recent years, the reproducibility of this semiquantitative measurement has been of great concern, and changes in SUV after therapy have been used to evaluate its efficacy (14). Therefore, such an effect of ARB treatment on renal function may consequently change SUV in 18F-FDG PET images and cause difficulties with diagnosis in clinical practice.
In agreement with our data, an increase in serum creatinine level after initiation of ACEI or ARB therapy was also commonly found in clinical trials, generally occurred within 2 wk, and was not associated with long-term harm (2). At the same time, 18F-FDG PET is also widely applied in oncology, cardiology, and neurology (6). There is a high probability that 18F-FDG PET was performed on patients receiving ARB or ACEI treatment. These lines of evidence suggest that the concomitant increase in blood 18F-FDG level may also be found in ARB- or ACEI-treated patients undergoing 18F-FDG PET.
From clinical data, the decrease in GFR associated with the initiation of renin angiotensin aldosterone system inhibition was reported to be reversible with discontinuation of ACEI or ARB therapy (15). In concordance with this observation, in the present study a brief discontinuation of ARB treatment reversed the 18F-FDG levels in the blood and organs to baseline levels (Fig. 2B) and reversed 18F-FDG excretion in the kidneys (Fig. 3). These findings indicate that a brief cessation of ARB treatment may be a potential method to avoid the effects of ARBs on 18F-FDG PET imaging in a clinical setting.
In the present study, we did not directly measure GFR in ARB-treated mice, but the elevated plasma creatinine levels in our mice indicate a decrease in GFR. The decreased GFR consequently may delay 18F-FDG elimination and result in high blood levels of 18F-FDG. As evidence of the close relationship between 18F-FDG excretion and renal function, in the present study the 18F-FDG level in the blood concomitantly changed with plasma BUN and creatinine levels (Supplemental Fig. 2) and correlated positively with plasma BUN (r = 0.48, P < 0.01) and creatinine levels (r = 0.61, P < 0.01) (Fig. 1).
In this study, serum creatinine levels were lower than 0.7 mg/dL even in mice treated with high-dose telmisartan. A creatinine level of 0.7 mg/dL is still within the reference range for mice. In patients, ARB or ACEI treatment increases the serum creatinine level to approximately 3 mg/dL (2), whereas the reference range for humans is from 0.6 to 1.2 mg/dL. Accordingly, the effects of ARBs on the animals in our experiments may not be unusual in humans. Moreover, we found that low-dose ARB treatment also significantly increased 18F-FDG activity in the blood without severely changing creatinine and BUN levels. This observation may further increase the probability of our finding that 18F-FDG activity increases in the blood of patients given ARBs.
Many patients who received ARB therapy show reversed renal function. In patients with renal problems, this is generally defined as the improvement of proteinuria or albuminuria (16). Reversed renal function, however, is commonly accompanied by an increased serum creatinine level. ARBs, particularly at high doses, decrease intraglomerular pressure, simultaneously improving proteinuria or albuminuria, which results in the renal protective effect (17). These patients commonly show an increased serum creatinine level, which is not considered a sign of renal insufficiency (18). The mechanisms underlying the decreased rate of urinary excretion of proteins and 18F-FDG are considered to be the same, that is, the decrease in intraglomerular pressure caused by ARB treatment.
The reversible reduction of 18F-FDG excretion by ARB may also suggest the utility of this agent for optimization of urinary 18F-FDG activity during PET imaging. Because an accumulation of 18F-FDG in the urine interferes with visualization of pelvic and abdominal abnormalities, radiologists or oncologists occasionally need to empty patients’ bladders before the PET scan (19) or even to retrograde-irrigate the urinary bladder with saline and a Foley catheter before or during PET data acquisition (20,21). To avoid such an invasive procedure, doctors also have used diuretics in an effort to reduce 18F-FDG activity in the urinary tract and reported improved diagnostic accuracy for 18F-FDG PET in abdominopelvic malignancies (12). Several experimental studies have also been performed to examine other agents for this purpose (22,23). The effective and reversible reduction of 18F-FDG excretion by telmisartan administration in the present study may indicate the usefulness of ARB for urinary 18F-FDG activity reduction, which may be helpful in the discrimination of pathologic from physiologic 18F-FDG uptake in abdominopelvic regions.
In the present study, a high-fat diet was used to make the status of the animal model close to that of patients who receive ARB. C57BK/6J mice on a regular research diet show a low serum low-density lipoprotein cholesterol level of about 20 mg/dL, which may create concerns about hypolipidemia in human beings (24). In contrast, in C57BK/6J mice fed a high-fat diet, the serum low-density lipoprotein cholesterol level elevates to about 75 mg/dL, which is closer to that of a human being (24). Body weight was lower in high-dose telmisartan-treated mice than in control mice on the same high-fat diet, probably because of the antiobesity effect of telmisartan (25).
As a limitation of the present study, we did not measure the 18F-FDG urinary excretion level, which may directly indicate whether the elimination of 18F-FDG is affected. We did, however, visually evaluate 18F-FDG excretion by autoradiography of kidney sections (Fig. 3). From these images, we made several observations. First, before ARB treatment, 18F-FDG markedly accumulated in the inner part of the medulla, which contains the loop of Henle and collecting duct. This finding indicates that, in the absence of ARBs, 18F-FDG is incompletely reabsorbed in proximal tubules and eliminated through the urinary system. Second, in the case of ARB treatment, the intensive accumulation of 18F-FDG in the inner part of the medulla markedly decreased to be as low as that in the renal cortex, which contains the renal corpuscles, proximal tubules, and distal tubules. This finding indicates the attenuated 18F-FDG excretion was caused by ARB treatment. Third, 3 wk after treatment cessation, the marked accumulation of 18F-FDG in the inner part of the medulla was reversed, and the accumulation pattern was similar to that before the ARB treatment. This finding suggests that the 18F-FDG excretion impaired by ARB treatment is reversible and that a short period of discontinuation may be a potential method to avoid this effect.
Taken together, our findings in this experimental study suggest that blockade of the renin angiotensin aldosterone system by ARB may affect the distribution of 18F-FDG by delaying its elimination. The increase in the background level of 18F-FDG, as well as the overestimation of SUV in target tissues, may adversely affect the accurate assessment of 18F-FDG PET images of ARB-treated patients. A brief treatment cessation may improve image quality. Nevertheless, there may be species differences between mice and humans, and our findings still need to be clinically confirmed.
CONCLUSION
Our data demonstrated that telmisartan administration may deteriorate renal function and cause an elevated level of 18F-FDG activity in the blood and other organs. The deterioration can be recovered by cessation of the treatment. The findings indicate the need for attention in the assessment of 18F-FDG activity in patients with telmisartan treatment and renal insufficiency.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. This study was performed through special coordination funds for promoting science and technology from the Japanese Ministry of Education, Culture, Sports, Science and Technology. This research was also supported in part by a grant-in-aid for general scientific research from the Japan Society for the Promotion of Science. No other potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank the staff members of the Department of Nuclear Medicine and Central Institute of Isotope Science, Hokkaido University, and the Facility of Radiology, Hokkaido University Medical Hospital, for supporting this work.
Footnotes
Published online Jul. 5, 2013.
- © 2013 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication November 2, 2011.
- Accepted for publication February 26, 2013.