Abstract
The success of liver transplantation (LT) for hepatocellular carcinoma (HCC) is enhanced by careful patient selection on the basis of the Milan criteria. The criteria are traditionally assessed by contrast CT, which is known to be affected by structural or architectural changes in cirrhotic livers. We aimed to compare dual-tracer (11C-acetate and 18F-FDG) PET/CT with contrast CT for patient selection on the basis of the Milan criteria. Methods: Patients who had HCC and had undergone both preoperative dual-tracer PET/CT and contrast CT within a 1-mo interval were retrospectively studied. They then underwent either LT (n = 22) or partial hepatectomy (PH) (n = 21; HCC of ≤ 8 cm). Imaging data were compared with data from postoperative pathologic analysis for accuracy in assessment of parameters specified by the Milan criteria (tumor size and extent, vascular invasion, and metastasis), TNM staging, and patient selection for LT. Results: Dual-tracer PET/CT performed equally well in both LT and PH groups for HCC detection (94.1% vs. 95.8%) and TNM staging (90.9% vs. 90.5%). Contrast CT performed reasonably well in the LT group but not in the PH group for HCC detection (67.6% vs. 37.5%) and TNM staging (54.5% vs. 28.6%). In the LT group, the sensitivity and specificity of contrast CT for patient selection on the basis of the Milan criteria were 43.8% and 66.7%, respectively (comparable to values in the literature); the sensitivity and specificity of dual-tracer PET/CT were 93.8% and 100%, respectively (both Ps < 0.05). From the surgeon’s perspective, we tended to perform transplantation for patients with higher diagnostic certainty (stricter CT criteria) because of a shortage of donor grafts. Patients who were not transplant candidates usually underwent up-front hepatectomy without the benefit of reassessment contrast CT, resulting in lower accuracies for the PH group. The overall sensitivity (96.8%) and specificity (91.7%) of dual-tracer PET/CT for patient selection for LT were significantly higher than those of contrast CT (41.9% and 33.0%, respectively) (both Ps < 0.05). Sources of error for contrast CT were related to cirrhosis or previous treatment and included difficulty in differentiating cirrhotic nodules from HCC (39%) and estimation of tumor size (14%). Overstaging of vascular invasion (4.6%) and extrahepatic metastases (4.6%) was infrequent. The rate of false-negative results of dual-tracer PET/CT was 4.7%. Conclusion: Dual-tracer PET/CT was significantly less affected by cirrhotic changes than contrast CT for HCC staging and patient selection for LT on the basis of the Milan criteria. The inclusion of dual-tracer PET/CT in pretransplant workup may warrant serious consideration.
Surgical treatment offers the best chance of cure for patients with hepatocellular carcinoma (HCC). Liver resection is a safe option in experienced hands. However, the tumor recurrence rate at 5 y after partial hepatectomy (PH) can be as high as 80% (1–3), and patients with limited functional hepatic reserve are not considered suitable candidates for the procedure. Liver transplantation (LT) is thus regarded as the ultimate solution for these patients.
Initial trials of LT for HCC in the 1980s did not have a satisfactory outcome, with 5-y survival rates of 18%–40%. The low success rates were largely attributed to inappropriate patient selection and a long waiting time because of graft scarcity (4–7). In 1996, Mazzaferro et al. (8) proposed the Milan criteria to maximize the benefit of orthotopic LT. They proposed a set of selection criteria on the basis of the relationship between small HCCs and better patient survival after LT. They found that patients with radiologic evidence of a single tumor with a diameter of 5 cm or less or a maximum of 3 tumors each with a diameter of 3 cm or less had a 5-y survival rate of 75% and a disease-free survival rate of 83%. Their findings were later confirmed by other transplant research groups (9). Although there are individual differences in these criteria, there is agreement that tumor number and size are the key factors that affect patient survival.
In all studies of criteria for patient selection for LT, the imaging tool used for the selection of patients has been either CT or MRI. It is well known, however, that their sensitivity for the detection of HCC is suboptimal in the presence of architectural distortion caused by severe cirrhosis in pretransplant patients, mostly reported to be about 60%–70% (10,11). In recent years, double-contrast MRI with both gadolinium and superparamagnetic iron oxide has been reported to have significantly improved the detection of HCC in cirrhotic livers, with a sensitivity of 75%–80% (12–14). Besides CT and MRI, PET has also been used.
In the past few years, dual-tracer PET/CT with 11C-acetate (11C-ACT) and 18F-FDG has been found to be useful in the evaluation of primary and metastatic HCC disease (15–17). Because these 2 tracers are biochemical probes of HCCs and are complementary to each other, according to tumor cellular differentiation, they can not only maximize sensitivity for the detection of malignant lesions but also identify tumors with better differentiation and better prognosis (18). Previous studies (15,18) suggested that the capacity of dual-tracer (11C-ACT and 18F-FDG) PET/CT to perform a whole-body metastatic survey (including bones) makes it the most comprehensive modality for staging compared with conventional radiologic imaging methods. On the basis of these potential advantages, we performed the present study to evaluate the accuracy of dual-tracer PET/CT for HCC staging and patient selection for LT on the basis of the Milan criteria and compared the results with those of contrast CT.
MATERIALS AND METHODS
Patients
Patients who had undergone LT or PH (largest lesion size, ≤8 cm) for HCC at the Department of Surgery, Queen Mary Hospital, The University of Hong Kong, between March 2004 and March 2010 were retrospectively reviewed. As a general guideline, patients with satisfactory liver function were offered liver resection as the first treatment. Patients who had poor liver function that was not expected to tolerate liver resection were considered for LT.
For comparison of the accuracy of dual-tracer PET/CT with that of contrast CT for HCC staging and patient selection for LT on the basis of the Milan criteria, all patients meeting the following 3 conditions were enrolled in the study. First, the patients had received preoperative assessment by both triphasic CT of the abdomen and dual-tracer PET/CT, and the time interval between the 2 imaging sessions had to be less than 1 mo. Second, the patients had not received any treatment between preoperative imaging (dual-tracer PET/CT and contrast CT) and surgery. For patients who had undergone PH, the third condition was that there should be no tumor involvement of resection margins, as documented by postoperative histopathologic analysis.
All patients provided verbal and written consent after being given clear instructions and explanations about the purposes and details of this study, which was approved by the institutional ethics committee (equivalent to an institutional review board) at Hong Kong Sanatorium and Hospital; study approval was also obtained from The University of Hong Kong.
Forty-three patients (34 men and 9 women; age range, 30–77 y; median age, 61 y) satisfied the inclusion criteria; 22 had LT (age range, 30–64 y; median age, 55 y), and 21 had PH (age range, 42–77 y; median age, 65 y). Thirty-seven patients were hepatitis B virus carriers, and 3 were hepatitis C virus carriers. The patients’ clinical and biochemical data are shown in Table 1. There were no significant differences in age, sex ratio, Child–Pugh grade, major organ biochemistry, α-fetoprotein concentration, and hepatitis virus status between the 2 groups of patients. The median time intervals between imaging and surgery were 35 d for CT and 21 d for PET/CT. Five patients in the LT group and 1 patient in the PH group had received bridging treatment by transcatheter arterial chemoembolization (TACE) before preoperative assessment by contrast CT and dual-tracer PET/CT. One patient had contrast CT and dual-tracer PET/CT before and after TACE.
Clinical and Biochemical Characteristics of Patients Who Had Undergone PH and LT
Dual-Tracer PET/CT and Contrast CT
The dual-tracer PET/CT protocol was previously described (15,16). All patients fasted for at least 6 h, and the blood glucose concentration was determined before the injection of PET radiopharmaceuticals (all had glucose levels of <8 mmol/L). 11C-ACT (440–590 MBq; 7.4 MBq/kg of body weight) was administered intravenously, and limited whole-body (from base of skull to upper thigh) imaging was performed at 20 min after injection. Data acquisition with an integrated in-line PET/CT scanner (Biograph LSO or Biograph 16 LSO HI-REZ; Siemens) began with CT (no contrast agent, 130 kV, 110–115 mA, 2-mm pitch, and 1-s tube rotation); CT was followed by PET with a 2-min emission acquisition time and a 16.2-cm axial field of view per position. About 15 min after the completion of 11C-ACT imaging, 330–520 MBq (6.3 MBq/kg of body weight) of 18F-FDG were injected intravenously. PET/CT imaging with the same positions and acquisition settings began at 60 min after 18F-FDG administration.
All CT studies were performed with a multidetector CT scanner (Light Speed 16; GE Healthcare) and standard unenhanced and 3-phase contrast-enhanced protocols (collimation, 1.5–2.0 mm; image reconstruction interval, 5 mm) for dynamic evaluation of the liver. Enhanced images were acquired after intravenous contrast agent injection (Omnipaque 300; 2 mL/kg; GE Healthcare). Guided by an early arterial phase automatically acquired with a 180–Hounsfield unit increase in attenuation, with reference to the abdominal aorta at the level of the liver dome, a late arterial phase followed 12 s later, and then a portal venous phase followed 40 s later. Delayed-phase imaging was performed when better delineation of lesion extent was needed or to check for capsular enhancement.
Interpretation According to Milan Criteria
In the present study, a patient was classified as either satisfying or not satisfying the Milan criteria for transplantation (a single HCC lesion of ≤5 cm or a maximum of 3 HCC lesions of ≤3 cm each, no macroscopic vascular invasion, and no extrahepatic metastasis) on the basis of preoperative imaging with dual-tracer (11C-ACT and 18F-FDG) PET/CT or contrast CT.
An intrahepatic lesion (liver or intrahepatic tumor thrombus) on PET images was considered to be an HCC on the basis of focally increased 11C-ACT or 18F-FDG metabolism and a well-defined margin compared with the background liver parenchyma, as visually judged by 3 nuclear medicine physicians (each with 7–10 y of experience in dual-tracer PET/CT). They read the images independently before reaching a consensus on the final diagnosis, which was supported by a semiquantitative evaluation (lesion-to-liver maximum standardized uptake value [SUV] ratio, >1.20). The lesion boundary was delineated in 3 dimensions automatically by the second derivative “PET edge” computational method with MIMContouring V4.1.8 commercial software (MIM Software, Inc.). The longest diameter of the lesion calculated by this computational algorithm was regarded as the “functional” lesion size on PET.
An extrahepatic lesion (extrahepatic tumor thrombi or metastasis) was assessed with the same evaluation methods, except that only the maximum SUV of the lesion was measured (the ratio was not necessary in the absence of background liver activity), and was considered to be an HCC if the maximum SUV was greater than 2.5 on either 18F-FDG or 11C-ACT PET images.
CT studies were interpreted independently by experienced radiologists without prior knowledge of the dual-tracer PET/CT findings. A lesion was considered positive for HCC if it showed contrast enhancement during the arterial phase and washout with isoattenuation or hypoattenuation during the portal venous or delayed equilibrium phase. A mosaic pattern and delayed capsular enhancement were also taken as ancillary signs of a positive diagnosis. The “physical” lesion size was regarded as the longest orthogonal diameter on the transaxial or reconstructed sagittal or coronal CT images. Other information, such as vascular tumor involvement and metastases, was likewise recorded independently.
The imaging findings were compared with the results of histopathologic analysis of explanted livers and resected liver and extrahepatic specimens (lymph nodes, vessels, and peritoneum), if any, because histopathologic analysis was used as the reference standard. Specific parameters recorded for each patient were diagnosis of individual liver lesions (HCCs, dysplastic nodules, and regenerative nodules), size, location (liver segment), number of lesions, tumor cellular grade or differentiation, vascular thrombosis with or without tumor involvement, nontumor liver tissue status, and extrahepatic metastasis. A lesion seen on contrast CT or PET/CT was considered to be a true-positive lesion if it was confirmed to be HCC by microscopic histopathologic analysis and was in the same location and segment indicated by gross pathology.
Statistical Analysis
Quantitative comparisons between contrast CT and 18F-FDG PET/CT, 11C-ACT PET/CT, and dual-tracer PET/CT were made with the χ2 test for the following aspects: sensitivity for the detection of HCCs and small (≤2-cm) HCCs, HCC staging (TNM system; (19)), and patient selection for LT according to the Milan criteria. P values of less than 0.05 signified statistical significance. Histopathologic analysis was used as the reference standard.
RESULTS
Pathologic Examination
The diagnosis of HCC was confirmed in all patients, and each patient had at least 1 HCC lesion found by postoperative pathologic analysis.
LT Group
Twenty-two patients in the LT group had a total of 34 HCC lesions with a median tumor size of 2.5 cm (range, 1.0–8.0 cm). Cirrhosis, dysplastic nodules, and regenerative nodules were found in all explanted livers. One patient had intrahepatic portal vein tumor thrombosis. All specimens were examined, including 4 specimens (a 2.0-cm portal node, a peritoneal biopsy at a location with significant mesenteric stranding, and 2 portal venous thrombi) from 4 patients with suspect findings on CT, and no extrahepatic metastasis was found. Histopathologic evidence obtained after LT showed that 16 patients (72.7%) met the Milan criteria and that 6 patients (27.3%) did not. Among the latter 6 patients, 3 had tumors larger than 5 cm (6.5, 7, and 8 cm) and 3 had multicentric HCC, each with 1 lesion larger than 3 cm (3.5, 4, and 5 cm). According to the TNM staging system, 13 patients (59.1%) were at stage I, 8 patients (36.4%) were at stage II, and 1 patient (4.5%) was at stage III.
PH Group
Twenty-one patients in the PH group had a total of 24 HCC lesions with a median tumor size of 2.8 cm (range, 1.0–7.5 cm). One patient had vascular tumor invasion of a portal vein branch. All resected liver specimens showed moderate cirrhotic features, with dysplastic and regenerative nodules. On the basis of the findings for the resected liver specimens and the assumption that all tumors were removed (all with clear resection margins), 15 patients (71.4%) met the Milan criteria and 6 patients (28.6%) did not. Each of the 15 patients who met the Milan criteria had only 1 tumor, and these tumors were smaller than 5 cm (range, 1–4.5 cm). Of the 6 patients who did not meet the criteria, 3 had tumors larger than 5 cm (5.4, 7.0, and 7.5 cm); 2 had multicentric HCC, with 1 lesion being larger than 3 cm (3.3 and 3.5 cm); and 1 had a tumor measuring 2.5 cm and tumor invasion of a portal vein branch. According to the TNM staging system, 16 patients (76.2%) were at stage I, 3 patients (14.3%) were at stage II, and 2 patients (9.5%) were at stage III.
When the 2 groups were combined, there were a total of 58 HCC lesions, including 23 small HCC lesions (1–1.5 cm: n = 11; >1.5–2 cm: n = 12; median size, 1.5 cm). Twenty-seven HCC lesions, including 15 small HCC lesions (<2 cm), were well differentiated; 31 HCC lesions, including 8 small HCC lesions, were moderately differentiated. No lesion was classified as poorly differentiated by histopathologic analysis.
Preoperative CT and PET/CT Imaging
Detection of Malignant Lesions
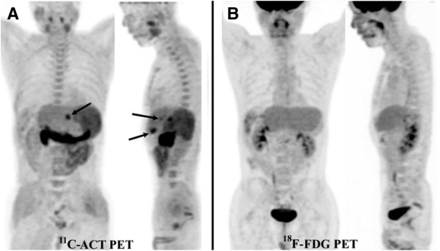
The lesion- and patient-based sensitivity for the detection of malignant lesions by triphasic contrast CT and 18F-FDG, 11C-ACT, and dual-tracer PET/CT in the LT group is shown in Table 2. In the LT group, 11C-ACT PET/CT was more sensitive than 18F-FDG PET/CT and contrast CT for the detection of HCC (all Ps < 0.05). Figure 1 shows a typical patient with 3 liver nodules avid for 11C-ACT but not 18F-FDG; each was confirmed to be a well-differentiated HCC smaller than 3 cm in explanted liver. Two patients with false-negative 11C-ACT PET/CT results had received bridging treatment by TACE before preoperative imaging. The pathology of the explanted livers of these 2 patients showed significant tumor necrosis and a small amount of viable tumor tissue. The sensitivity for the detection of malignant lesions in the PH group is also shown in Table 2. Again, 11C-ACT PET/CT was more sensitive than contrast CT and 18F-FDG PET/CT (all Ps < 0.05).
Maximum-intensity-projection PET images of typical patient who had HCC and met Milan criteria. Three hypermetabolic liver lesions (arrows), each smaller than 3 cm, in left lobe (after right hepatectomy) were avid for 11C-ACT (A) but not 18F-FDG (B). There was no extrahepatic metastasis.
Sensitivities of PET/CT and Contrast CT for Detection of Malignant Lesions*
Contrast CT was found to have an overall lesion-based sensitivity of 55.2% (Table 2), with individual detection sensitivities of 67.6% in the LT group and 37.5% in the PH group (P < 0.05 for the 2 groups). There was no significant difference in the sensitivity of 11C-ACT and 18F-FDG PET/CT between the 2 groups (both Ps > 0.05). The overall sensitivity of 18F-FDG PET/CT was low (32.8%), as already shown by an earlier report (16) indicating that early HCCs were more often tumor cells of a younger generation and with better differentiation and that their metabolic avidity favored 11C-ACT instead of 18F-FDG.
To evaluate the ability of the present study to delineate small HCCs, we analyzed a subgroup of small tumors (1–2 cm; mean ± SD, 1.6 ± 0.28 cm). The sensitivity for the detection of small HCCs by 11C-ACT PET/CT was 87.0%, which was comparable to its overall sensitivity (93.1%) (P > 0.05) (Table 2). The sensitivities for the detection of small HCCs by 18F-FDG PET/CT and contrast CT were 17.4% and 43.5%, respectively; these sensitivities were significantly lower than their overall sensitivities (both Ps < 0.05). Almost two-thirds of the small HCCs were well differentiated (15/23; 65.2%). A total of 12 dysplastic nodules (≥1 cm; mean ± SD, 1.5 ± 0.34 cm) were identified by postoperative pathologic analysis. 18F-FDG PET/CT and 11C-ACT PET/CT yielded true-negative results for all dysplastic nodules (specificity, 100%). Contrast CT yielded false-positive results for 2 dysplastic nodules (1.5 and 2.0 cm) with mild arterial-phase contrast enhancement; they were misdiagnosed as small HCCs (specificity, 83.3%).
11C-ACT PET/CT correctly identified 1 patient in the LT group as having tumor thrombi along an intrahepatic portal vein and a main portal vein (seen as 11C-ACT–avid hypermetabolic lesions), but the results of contrast CT were indeterminate because of the lack of contrast enhancement within the thrombi. 11C-ACT PET/CT yielded true-negative results for 4 patients for whom extrahepatic metastasis was suggested by CT (2 with vascular tumor thrombi, 1 with an enlarged portal node, and 1 with a mesenteric metastasis). Both 11C-ACT and 18F-FDG PET/CT and contrast CT revealed no macroscopic vascular invasion or extrahepatic metastasis in the PH group, but there was pathologic evidence of vascular invasion in 1 patient’s resected liver specimen (all types of imaging yielded false-negative results).
TNM Staging and Patient Selection for LT According to Milan Criteria
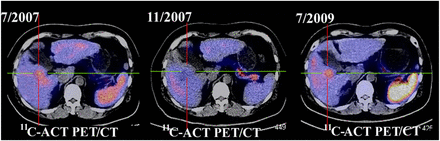
Table 3 shows the accuracy of the 4 imaging modalities (triphasic contrast CT, 11C-ACT PET/CT, 18F-FDG PET/CT, and dual-tracer PET/CT) for TNM staging (19) in the 2 groups. 11C-ACT PET/CT was more accurate for staging than contrast CT and 18F-FDG PET/CT in both groups of patients (all Ps < 0.05). Figure 2 shows that CT had difficulty in differentiating dysplastic nodules from small HCCs in a typical patient with cirrhosis. The differentiation might have been more uncertain in patients who had undergone TACE treatment. However, changes after TACE seemed to have little effect on PET images (Fig. 3). Although, on the basis of the contrast CT findings after TACE, this patient did not meet the Milan criteria, TACE should have had at least a partial effect, as reflected by the improved clinical and biochemical parameters. With concordant findings from PET/CT and other favorable clinical factors, including the availability of a graft, the patient subsequently underwent LT. The results of pathologic analysis agreed with the PET/CT findings, indicating that this patient met the Milan criteria.
CT showed mildly contrast-enhanced nodule in segment IVb impinging on gallbladder, suggestive of HCC (arrowhead), with no abnormal metabolism on 11C-ACT PET/CT (or on 18F-FDG PET/CT; data not shown). Instead, 11C-ACT–avid lesion (arrow) was found in segment V. Results of pathologic analysis for excised liver confirmed well-differentiated HCC (3.6 cm) in right lobe (segments V and VI) and dysplastic nodule (2.0 cm) in left lobe (segment IVb), adjacent to gallbladder.
(A) Multicentric HCC recurrence in left lobe of liver in patient after right hepatectomy. (B) 11C-ACT PET after TACE showed successful downstaging, with 2 lesions left (arrows), thus within Milan criteria. (C) CT showed 6 or 7 contrast-enhanced lesions (red arrows, CT 1–CT 5) and enlarged portal node (red circle, CT 6), hence beyond Milan criteria. In excised liver, 2 small HCCs (2.4 and 2.7 cm), concordant with 2 11C-ACT–avid lesions, and a few necrotic nodules without malignant cells were found. Portal node was found negative for metastasis by pathologic analysis and 11C-ACT PET/CT.
TNM Staging Accuracies of PET/CT and Contrast CT for Patients with HCC*
Figure 4 shows the only lesion suspected of being a high-grade dysplastic nodule on the basis of mild 11C-ACT avidity (lesion-to-liver maximum SUV ratio, 1.15). PET performed 4 mo later revealed normalized metabolism, suggestive of regression (the patient was receiving antiviral treatment). Nonetheless, PET performed 20 mo thereafter revealed a well-defined 11C-ACT–avid nodule in the same location. Without knowledge of the PET findings, the pathologist who examined the explanted liver described this nodule as “having features of a well-differentiated HCC (1.8 cm) that was transformed from a high-grade dysplastic nodule.”
Carrier of hepatitis B virus in LT group with suspected liver nodule was evaluated and monitored for 2 y by dual-tracer PET/CT (3 times). 11C-ACT PET/CT image in July 2007 showed 11C-ACT–avid lesion with ill-defined margins in segment V, suggestive of early HCC or high-grade dysplastic nodule. Activity of lesion was normalized 4 mo later but reappeared in 2009.
Table 4 summarizes the abilities of PET/CT and contrast CT to correctly select patients for LT on the basis of the Milan criteria. Assessment was made in terms of 4 elements—lesion size, lesion number, intrahepatic vascular invasion, and extrahepatic metastasis. 11C-ACT PET/CT was significantly more accurate in patient selection than contrast CT and 18F-FDG PET/CT (all Ps < 0.05). On the basis of the results of pathologic analysis, the overall accuracies of 11C-ACT PET/CT for patient inclusion and exclusion were 93.5% (29/31 correctly identified as meeting the Milan criteria) and 91.7% (11/12 correctly identified as not meeting the Milan criteria), respectively. The incremental values of 11C-ACT PET/CT over contrast CT for patient inclusion and exclusion were 51.6% and 58.3%, respectively. Sources of error (nonexclusive) for contrast CT in patient inclusion and exclusion were indeterminate or negative conclusion in differentiating cirrhotic nodules (dysplastic nodules, regenerative nodules, or changes after TACE) from HCCs (39.5%; 17/43), inaccurate account of lesion size (14%; 6/43) or number (2.3%; 1/43), and overstaging because of a false-positive diagnosis of vascular (4.7%; 2/43) or extrahepatic (4.7%; 2/43) metastasis. Discordance between 11C-ACT PET/CT and pathologic findings was found for 3 patients with false-negative results; the results for 2 of these patients were determined to be negative by both tracers. These 2 patients had tumor necrosis with minimal residual tumor tissue after TACE.
Accuracies of PET/CT and Contrast CT in Selecting Patients for LT on Basis of Milan Criteria*
DISCUSSION
After the introduction of the conventional Milan criteria in 1996, the results of orthotopic LT improved substantially through careful patient selection (8,9). The objectives of the criteria are to include more patients with a good prognosis for LT and to prevent a poor prognosis by avoiding transplantation in patients who do not meet the criteria. The criteria enable objective patient selection and provide a fair system for allocation of the limited supply of liver grafts. With 10 y of experience, the Milan group achieved a patient survival rate of over 70% in 300 LTs by adopting the criteria (20). Equally good results were observed in the rest of the world when a similar principle was followed, although the criteria might have been expanded at some centers (21,22).
The Milan criteria feature 2 independent predictors of patient survival and disease recurrence—tumor size and tumor number. The most accurate measurement of tumor size and number is obtained by examination of pathologic specimens, but this information can be acquired only after transplantation. Contrast CT has been used as a standard assessment tool for this purpose, but its accuracy has not been greatly improved even by advanced multidetector CT with fast acquisition and computation capabilities (21,23). The typical triphasic pattern of arterial enhancement may not always be present in small HCCs on CT scans. On the other hand, true tumors are often obscured by a background of cirrhosis intermixed with regenerative and dysplastic nodules. Furthermore, other hypervascular lesions, such as a small hemangioma, an inflammatory nodule, or a focal arterioportal shunt, may mimic small HCCs. False-positive and false-negative nodules are common findings. It has been suggested that only 10%–30% of nodules smaller than 2 cm on the arterial phase of contrast CT represent HCCs in highly cirrhotic livers (24). On the other hand, it was reported that ultrasound imaging, CT, and MR imaging revealed hypervascularity in 34%, 52%, and 47% of HCCs of different grades and with a median size of 1.6 cm, respectively. MR imaging, especially dynamic gadolinium-enhanced MR imaging and double-contrast MR imaging, has been reported to be better than CT for the overall detection of HCCs (12–14,24,25). However, a Korean study showed that multidetector CT, superparamagnetic iron oxide MR imaging, and double-contrast MR imaging had comparable accuracies for assessing the appropriateness of patients for LT on the basis of the Milan criteria (12).
Tumor extension is often over- or underestimated (27%–50%) in cirrhotic livers; this was the case in the original study that proposed the Milan criteria (8,26,27). Several large series of studies on the sensitivity of CT (helical and multidetector CT) in patients with cirrhotic HCC treated by LT reported a sensitivity range of 60%–70% on a patient basis (23,28–30). Our result in the LT group was 73.3% (67.6% on a lesion basis), in agreement with the results in the literature. The only difference is the lower sensitivity (42.9%; 37.5% on a lesion basis) in the PH group.
We reviewed these studies and found 2 possible reasons for the results. The first reason is related to the CT diagnostic criteria. Some of these studies, in addition to the typical contrast enhancement pattern, included lesions without arterial-phase enhancement but with delayed-phase washout. Therefore, studies that adopted this wider margin for diagnosis also reported a large number of false-positive nodules (up to 59%) (30). The second reason is based on the first reason; the stricter criteria in the present study tended to include patients with a greater certainty of diagnostic accuracy but also tended to exclude more patients with less certainty. In view of the severe shortage of liver grafts in Hong Kong, we embrace a more cautious attitude so that preference is given to patients who are expected to have more definitive CT results.
Patients who are not LT candidates because of various individual factors will undergo PH as a definitive treatment as soon as the surgery can be arranged. Some patients with less definitive CT findings will also undergo tumor resection as long as the clinical diagnosis is highly suggestive of HCC. Therefore, we speculate that a “preselection” element might have contributed partly to the lower performance of contrast CT in the PH group.
Patient selection on the basis of the Milan criteria added another set of conditions to the criteria for lesion detection. CT findings agreed with pathologic findings for 43.8% of the patients in the LT group and for 40% of the patients in the PH group. These rates are similar to that reported in a German study (41%) (27). The performance of 11C-ACT PET/CT in the LT group was similar to that in the PH group, a result that is not surprising because it is relatively independent of benign anatomic or structural variations. As shown in Tables 2–4, sensitivity for the detection of malignant lesions, staging accuracy, and consistency with the Milan criteria were similar (>90%) in the 2 groups when dual-tracer PET/CT was used. Therefore, the data suggested that patients in the PH group could have been given equal consideration for LT had the dual-tracer PET/CT findings been used as the criterion evaluating tool. This approach would definitely affect management decisions. Nonetheless, more data are needed for further validation.
In the last decade, Ho et al. studied the complementary nature of 2 biochemical PET tracers, 11C-ACT and 18F-FDG, for the detection of primary and metastatic HCCs (15,16). They found that de novo synthesis of free fatty acids is upregulated in well-differentiated HCCs and that acetate (11C-ACT), a precursor of acetyl-coenzyme A, may be the preferred metabolic substrate. As HCC cells become more malignant and dedifferentiated, their biochemical substrates for growth also progressively shift to 18F-FDG, a marker of glycolysis. The sensitivity of an individual tracer for the detection of malignant lesions is thus dependent on tumor cell differentiation, and the dual tracers are complementary. The sensitivity of 11C-ACT PET alone for the detection of malignant lesions is approximately 87% for HCCs of intermediate and small sizes. Because early or small HCCs tend to be well differentiated, 11C-ACT is the more important tracer for PET imaging. In the present study, the sensitivity of 11C-ACT PET/CT for the detection of small HCCs (1–2 cm) was 87.0%. This rate is similar to those reported in previous studies, but it was derived from a different, independent group of patients (31). It was also significantly higher than those achieved by 18F-FDG PET/CT (17.4%) and contrast CT (43.5%) in the present study.
Our results showed that regenerative nodules were not avid for either of the 2 tracers and that they could be distinguished from HCCs. Dysplastic nodules were frequently found in pathologic specimens, but only 1 lesion was retrospectively suspected of being a high-grade dysplastic nodule that showed mild 11C-ACT avidity before LT (Fig. 4). More data are needed to evaluate the relationship between 11C-ACT and high-grade dysplastic nodules. Previously published data suggested that structural changes secondary to cirrhosis do not affect the PET diagnosis as much as they do the CT diagnosis.
Vascular invasion and histopathologic grading are 2 other predictors of outcomes for LT in patients with cirrhotic HCC. These have been regarded as providing valuable prognostic information but otherwise are not available until after surgery. With dual-tracer PET/CT, these parameters are valuable for preoperative treatment planning (18). Although 11C-ACT was shown to have superior sensitivity for the detection of malignant lesions throughout the present study, the complementary role of 18F-FDG should not be underestimated, because an earlier report suggested that some metastatic HCC lesions may dedifferentiate even if the primary HCC tumor initially comprises 11C-ACT–avid, well-differentiated cell types (15). Therefore, 18F-FDG is needed for a complete assessment of all of the Milan criteria (metastasis). Moreover, 18F-FDG, as a marker of dedifferentiated HCC tumor pathology, has been shown by other researchers to be a predictor of tumor recurrence and a less favorable outcome after transplantation (32).
In the present study, we purposely included a group of recipients of PH for comparison. We assumed that there was no HCC disease in the unresected livers—the only part of the study without pathologic proof (but contrast CT and PET/CT findings were negative). Although recurrence was seen in some of these patients during follow-up, they were not considered different from other PH patients in whom recurrence was attributed to de novo metachronous tumor development, occult tumor seeding, or micrometastasis via portovenous sinuses or shunts and not to suboptimal preoperative imaging assessment.
Another limitation is that the synthesis of 11C-ACT requires technical expertise and an on-site cyclotron. With significant improvements in PET and cyclotron technologies in the past decade, many “baby cyclotrons” have become available and affordable. Because 18F-FDG is far from being a universal tracer for all tumors, the complementary use of a good tracer, such as 11C-ACT, for liver cancer should be encouraged.
CONCLUSION
This is the first study to use dual-tracer PET/CT for the evaluation of patients with HCC in a setting in which the Milan criteria are used to select patients for LT. The basic principle underlying these criteria is the ability of imaging to accurately define the true stage of disease. In the presence of chronic and cirrhotic liver disease, an imaging technique that is not so affected by the structural changes of the background parenchyma is needed. The results of the present study confirmed that dual-tracer PET/CT works in keeping with this principle because of the use of a pair of functional probes that are known to be less affected by structural changes and not compromised by size, that have complementary biochemical sensitivities to cover a wide range of tumor cells with different degrees of differentiation or dedifferentiation, and that can be used to perform a metastatic survey. All of these elements contribute to the potential of this technique for better patient selection. To understand the logistics, cost, and feasibility of this imaging technique in pretransplant workup for patients with HCC, large-scale research needs to be conducted.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. No other potential conflict of interest relevant to this article was reported.
Footnotes
Published online Jan. 15, 2013.
- © 2013 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication April 16, 2012.
- Accepted for publication September 14, 2012.