Abstract
The prediction of dopaminergic responsiveness in patients with parkinsonism is desirable for effective treatment strategies. We investigated whether striatal dopamine D2/D3 receptor (D2R) binding assessed by 123I-iodobenzamide SPECT is an independent predictor of dopaminergic responsiveness in patients with parkinsonism. Methods: Seventy-eight patients with clinically suspected atypical parkinsonian syndrome (APS) were prospectively recruited for imaging. To quantify striatal D2R binding, 123I-iodobenzamide SPECT datasets were subjected to an observer-independent, regions-of-interest analysis. A final clinical diagnosis of Lewy-body disease (LBD) or APS was made after a mean follow-up of 12 mo. On the basis of follow-up data, dopaminergic responsiveness was classified as 0 (none), 1 (transient), 2 (sustained mild), or 3 (sustained strong). Uni- and multivariate analyses of the relationship between treatment response, D2R binding, and confounding variables were conducted. Results: Sixty patients with clinically verified LBD (n = 28; 22/28 with Parkinson disease) or APS (n = 32), in whom dopaminergic responsiveness could be assessed (n = 19/13/15/13 in categories 0/1/2/3; 18 were excluded because of insufficient dosing), were included in the statistical analysis. Univariate analyses revealed that a sustained treatment response was significantly associated with higher D2R binding, clinical diagnosis of LBD, lower Hoehn and Yahr scores, and younger age. After multivariate correction of D2R binding for diagnosis, age, symptom duration, Hoehn and Yahr score, and dopaminergic pretreatment, no association was found between D2R binding and treatment response, either in the pooled group or in LBD or APS subgroups. Conclusion: Striatal D2R binding assessed by 123I-iodobenzamide SPECT does not provide additional predictive information about treatment response beyond other clinical variables, most notably the clinical diagnosis.
- [123I]IBZM single-photon emission computed tomography
- parkinsonism
- levodopa response
- atypical parkinsonian syndrome
According to current consensus criteria, poor responsiveness to levodopa is suggestive of atypical parkinsonian syndrome (APS) (1). However, up to 30% of patients diagnosed with multiple-system atrophy or progressive supranuclear palsy benefit from dopaminergic medication, particularly at early stages of the disease (2,3). Dopaminergics alleviate both motor and nonmotor symptoms and improve quality of life (4). Although neuroprotection trials have provided some evidence for disease-modifying effects of dopaminergic drugs (5,6), common side effects (i.e., psychosis, orthostatic hypotension, gastrointestinal disorders) may compromise the clinical condition (7). Moreover, the costs of unnecessary treatment need to be avoided. Thus, a reliable predictor of therapeutic efficacy is desirable.
Guidelines from the American Academy of Neurology recommend an acute levodopa challenge test to evaluate the sensitivity of target symptoms to levodopa (positive: >30% decrease in the score on the Unified Parkinson Disease Rating Scale III) (8). However, a negative test result is of limited validity in APS, since clear drug effects often require higher doses and longer treatment (9). In line with this observation, a systematic review yielded a higher diagnostic accuracy for chronic levodopa treatment than for acute challenge testing in the differential diagnosis of parkinsonian syndromes (10). Therefore, the national clinical guidelines for diagnosis and management in primary and secondary care of the National Institute for Health and Clinical Excellence discourage the use of acute levodopa challenge tests (11). Taking these reasons into account, assessment of the long-term treatment response should be the more favorable approach.
Functional integrity of postsynaptic dopaminergic neurotransmission is a prerequisite for effective dopaminergic responsiveness. A few studies have addressed postsynaptic integrity as a possible predictor of dopaminergic responsiveness by using 123I-iodobenzamide SPECT to assess striatal dopamine D2/D3 receptor (D2R) binding (12–15). Although these studies provided encouraging results in de novo and pretreated patients with parkinsonism, they did not account for possible confounding effects such as patient age, disease severity, and pretreatment. Furthermore, it is not known whether 123I-iodobenzamide SPECT is a predictor of treatment responsiveness beyond the final clinical diagnoses of APS or idiopathic Parkinson disease (PD), which are associated per se with reduced and preserved (sometimes even increased) D2R availability, respectively (16).
Considering this background, the present study investigated whether 123I-iodobenzamide SPECT can serve as an independent predictor of dopaminergic responsiveness in patients with clinically suspected APS beyond the final clinical diagnosis and other potentially confounding variables. The present study entailed the inclusion of all patients with suspected APS referred for imaging in clinical routine. The result was a clinically realistic sample in which patients with corticobasal degeneration, PD with dementia, and dementia with Lewy bodies were also included.
MATERIALS AND METHODS
Patients
All procedures were approved by the local ethics committee. Written informed consent was obtained from all participants. The present dataset stems from a large prospective study comparing the diagnostic accuracy of 18F-FDG PET and 123I-iodobenzamide SPECT in the differential diagnosis of parkinsonism (German Clinical Trials Register number DRKS00003613) (16). Briefly, between July 2008 and January 2011, 107 consecutive patients with suspected APS were referred for diagnostic imaging. Of these, 95 patients (88.8%) who fulfilled the inclusion criteria (i.e., suspected, but not yet verified, early-stage APS based on clinical symptoms and poor response to levodopa; Karnofsky score ≥ 40%) but not the exclusion criterion (i.e., relevant impairment or reduction of life expectancy caused by another disease) were prospectively recruited (16). Two board-certified neurologists specializing in movement disorders and masked to the imaging results made the final diagnosis in accordance with consensus criteria (1) after a follow-up of at least 6 mo, as previously described (16). Seventy-eight patients received a final clinical diagnosis of either LBD or APS. One neurologist, who was masked to the imaging results but had access to all relevant medical charts and follow-up data, rated dopaminergic responsiveness in these patients using a 4-step score (0/1/2/3: no/only transient/sustained mild/sustained strong response). Of note, clinical ratings of responsiveness also included phases of drug withdrawal (e.g., before SPECT imaging in the pretreated patients). To qualify as a nonresponder (including only transient responders; that is, scores 0–1), the patient had to receive a levodopa dose equivalent to at least 600 mg for at least 6 wk. In rare cases where levodopa side effects were dose limiting, the minimum dose for the acceptance of nonresponsiveness was 400 mg. Patients who did not fulfill these requirements were excluded from subsequent analyses. The levodopa dose equivalent was calculated as previously suggested (17,18).
123I-Iodobenzamide SPECT
Datasets were acquired on a dual-head SPECT system (E.CAM; Siemens) equipped with a low-energy, high-resolution collimator 90 min after the injection of 187.3 ± 10.0 MBq of 123I-iodobenzamide (GE Healthcare). The acquisition parameters were as follows: 120 projections of 30 s each, a radius of 13.5 cm, an energy window of 159 keV ± 15%, a 128 × 128 matrix, and a zoom factor of 1.23. Reconstruction was done by filtered backprojection (seventh-order Butterworth filter; cutoff, 0.36 Nyquist) with calculated attenuation correction (μ = 0.11/cm).
Volume-of-interest analyses including the bilateral striatum and frontal cortex (reference region; devoid of specific binding) were performed to assess regional tracer concentrations in an observer-independent fashion using commercial software (BRASS, version 3.5; Hermes Medical Solutions) (Fig. 1) (19). Specific striatal 123I-iodobenzamide uptake was estimated as the difference between total striatal and total frontal 123I-iodobenzamide uptake by assuming that the frontal cortex is devoid of D2R and that nondisplaceable tracer uptake is comparable in both regions. The ratio of specific striatal uptake to total (i.e., nondisplaceable) frontal uptake was calculated as a close approximation of the binding potential (BPND). This approach has been validated against pharmacokinetic reference-tissue models in a previous study using 142-min dynamic SPECT scanning (20). The average BPND of both striata served as an outcome measure that is directly proportional to the D2R density available for 123I-iodobenzamide binding (21).
Volume-of-interest analysis. Top row shows representative example of 123I-iodobenzamide study. Given are transaxial slices at height of striatum (voxel values were normalized to average frontal cortex uptake for standardized, semiquantitative display). Bottom row illustrates positioning of striatal and frontal volumes of interest by BRASS software after spatial normalization to 123I-iodobenzamide template (provided by software). Corresponding transaxial slices are depicted; coronal slice (right) gives extension of volume of interest in z direction.
Statistics
The commercial software package JMP 8 (SAS Institute Inc.) was used for statistical analyses. Between-group differences were assessed using the Student t test (continuous data), the Wilcoxon test (ordinal data), or the χ2 test (nominal data) (2-sided). In the case of multiple group comparisons, either a 1-factorial ANOVA with post hoc Tukey–Kramer HSD testing (continuous data) or a Kruskal–Wallis test with subsequent Wilcoxon testing (ordinal data; corrected for multiple comparisons) was used. Pearson and Spearman correlation coefficients were used to investigate correlations between continuous and ordinal variables, respectively. A linear regression model was used to calculate the residuals of D2R binding (BPND given by 123I-iodobenzamide SPECT) after adjusting for relevant variables. Finally, logistic regression was applied to explore independent predictors of dopaminergic responsiveness.
RESULTS
Patient Characteristics
Treatment Response
According to clinical follow-up data, patients were categorized in terms of dopaminergic responsiveness as follows: in 13 patients there was a sustained, strong response to dopaminergic treatment (category 3), whereas in 15 patients, treatment response was only mild but was clearly noticeable (category 2). Another 13 patients showed only a transient, mostly mild, response to dopaminergic treatment (category 1), whereas 19 patients showed no dopaminergic responsiveness. Four patients assigned to category 0 or 1 had dose-limiting side effects (two with hallucinations at levodopa dose equivalents of 400 and 550 mg, 1 with dizziness and leg edema at 400 mg, and one with syncope at 500 mg). Finally, 18 patients were excluded from the analysis because they either did not receive any dopaminergics or received an ineffective dose. Thus, a total of 60 patients were included.
Final Clinical Diagnoses
The final clinical diagnoses were as follows: 22 cases of PD, 6 of PD with dementia/dementia with Lewy bodies, 10 of multiple-system atrophy, 14 of progressive supranuclear palsy, and 8 of corticobasal degeneration. As previously described (16), we pooled PD and PD-with-dementia patients and classified them as the Lewy-body disease (LBD) group, whereas multiple-system atrophy, progressive supranuclear palsy, and corticobasal degeneration constituted the APS group. Responsiveness to treatment was not associated with subgroup diagnoses within the LBD or APS groups. At the time of SPECT imaging, there were no significant group differences in terms of sex, age, or symptom duration (Table 1). Time of follow-up did not differ between LBD and APS patients (Table 1), nor did it correlate with treatment responsiveness (Pearson r = 0.13, 0.28, and −0.23 for all, LBD, and APS patients, respectively; all P > 0.1). APS patients showed higher Hoehn & Yahr (H&Y) scores than did LBD patients (Table 1). The fraction of patients pretreated with dopaminergic medication before SPECT imaging was slightly higher in the LBD group. However, until the end of the follow-up period, APS patients were treated with higher maximum levodopa dose equivalents (Table 1).
Characteristics of Patient Groups
Dopaminergic Responsiveness and D2R Binding: Univariate Analyses
When all 60 patients were considered, D2R binding (given by BPND from 123I-iodobenzamide SPECT) was significantly higher in patients with an optimal treatment response (category 3; of note, including only LBD patients) than in patients with less favorable responses (categories 0–2; Table 2). Considering LBD patients only, ANOVA indicated a significant association between D2R binding and response categories. Although patients in category 3 showed the highest D2R binding, it failed to reach statistical significance on post hoc testing (Table 2). In APS patients, there was no association between D2R binding and treatment response categories. Similarly, for dichotomized response groups, a significant difference between responders (response categories 2 and 3) and nonresponders (response categories 0 and 1) was found in the combined sample of LBD and APS patients but not in each patient group alone. The area under the receiver operating characteristic curve (ROC AUC) for prediction of treatment response by D2R binding is 0.71 (using a BPND cutoff of 0.49, 123I-iodobenzamide SPECT correctly predicted treatment response in 42 of 60 [70%] patients).
Summary of Variables Across Groups of Response to Dopaminergic Treatment
However, D2R binding and treatment response might be influenced by several variables, the most likely being the clinical diagnosis, but possibly also patient age, symptom duration, and disease severity (assessed by H&Y score).
Clinical Diagnosis: Association with D2R Binding and Treatment Response
As previously shown (16), D2R binding is significantly lower (−17%) in APS than in LBD patients (Tables 1 and 2). A previous publication of ours (16) provides a detailed analysis of D2R binding in patient subgroups. Conversely, responsiveness to dopaminergic treatment is also highly significantly associated with the final clinical diagnosis (Table 2): although most APS patients (81%) showed no or only transient responsiveness, the opposite was true for LBD patients (79%). Thus, clinical diagnosis correctly predicted responsiveness to treatment (dichotomized) in 80% of patients (48/60).
D2R Binding: Association with Age, Symptom Duration, and Disease Severity
Regarding other potentially confounding factors, D2R binding was negatively correlated with age when all patients were collectively analyzed (Pearson r = −0.62; P < 0.0001)—an association that was driven by the LBD group (r = −0.81, P < 0.0001, −1.1% BPND decrease per year at a typical age of 65 y; APS group: r = −0.21, P = 0.25, −0.4%/y). D2R binding also showed a weak, albeit significant, negative correlation with symptom duration in LBD patients (r = −0.43, P = 0.024, −3.2%/y at a typical duration of 3.5 y) but not in APS patients (r = −0.14, P = 0.43, −1.3%/y) or all patients collectively (r = −0.23, P = 0.081). Furthermore, D2R binding was negatively correlated with H&Y score both in all patients (Spearman ρ = −0.56, P < 0.0001) and in LBD patients only (ρ = −0.60, P = 0.0008, −9.4% BPND decrease per H&Y score at a typical H&Y score of 3) but not in APS patients (ρ = −0.16, P = 0.37, −4.6%/score).
Treatment Response: Association with Age, Symptom Duration, and Disease Severity
In turn, patients who showed the best response to dopaminergic treatment (i.e., category 3) were younger and clinically less affected than those showing a less favorable response (Table 2; age: r = −0.21, P = 0.11; H&Y ρ = −0.44, P = 0.0004). When disease groups were considered separately, there was a trend toward lower age and disease severity in LBD patients showing an optimal response (age: ANOVA, P = 0.105, r = −0.27, P = 0.16; H&Y: Kruskal–Wallis, P = 0.074; ρ = −0.45, P = 0.016), whereas no difference was observed in APS patients (age: r = 0.18, P = 0.33, H&Y ρ = 0.02, P = 0.92). Symptom duration did not differ between response categories. For dichotomized response groups, responders showed a significantly lower H&Y score than did nonresponders in all patients and in the LBD patient group. Age and symptom duration did not differ between responders and nonresponders.
Association Between Dopaminergic Pretreatment and D2R Binding
Finally, dopaminergic treatment before SPECT scanning had to be considered as another variable that could possibly have a confounding effect on D2R binding. In line with this observation, D2R binding tended to be lower in pretreated LBD patients (BPND = 0.55 ± 0.10) than in those without any preceding dopaminergic treatment (BPND = 0.64 ± 0.07, P = 0.073; no difference in APS patients).
Independent Predictors of Dopaminergic Responsiveness
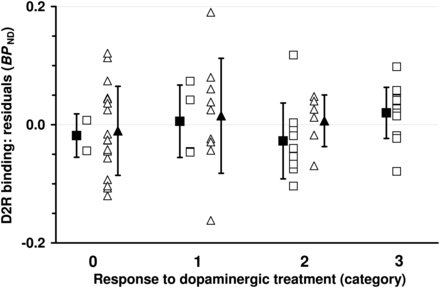
To distinguish the independent predictive value of D2R binding from the effects of final diagnosis, age, symptom duration, H&Y score, and dopaminergic treatment before SPECT, we first described D2R binding as a function of all other variables and then studied the association between the residuals (i.e., difference between expected and measured D2R binding values) and dopaminergic responsiveness. The residuals of D2R binding were calculated using a linear regression model: When all patients were considered as a collective, it was found that age (F1,54 = 15.23, P = 0.0003), final diagnosis (LBD/APS; F1,54 = 11.87, P = 0.0011), and symptom duration (F1,54 = 5.14, P = 0.027) each exhibited significant effects on D2R binding. Residuals of D2R binding did not correlate with response categories (Fig. 2; r = 0.12, 95% confidence interval, [−0.14;0.36], P = 0.37; t test, responders vs. nonresponders, P = 0.84).
Residuals of dopamine D2R binding (BPND; gained from 123I-iodobenzamide SPECT) across categories of response to dopaminergic treatment. Open squares and triangles represent individual data points from patients with LBD and APS, respectively. Filled symbols give mean values ± SD. Residuals are based on regression model including diagnosis (LBD/APS), age, symptom duration, H&Y score, and dopaminergic pretreatment before imaging (yes/no).
This analysis was then separately applied to the LBD group (significant predictors of D2R binding: age, F1,23 = 25.95, P < 0.0001, and symptom duration, F1,23 = 7.18, P = 0.013) and the APS group (no significant effect of predictors). Again, there were only weak, nonsignificant, associations between residuals of D2R binding and responsiveness to dopaminergic treatment (LBD: r = 0.17, 95% confidence interval, [−0.22;0.51], P = 0.38; APS: r = 0.08, 95% confidence interval, [−0.27;0.42], P = 0.65; t test, responders vs. nonresponders: LBD, P = 0.92, and APS, P = 0.93).
Multivariate Prediction of Treatment Response
Finally, logistic regression was used to predict responsiveness to treatment (dichotomized) including the final diagnosis, age, and disease severity (H&Y score). When all patients were collectively analyzed, only the final diagnosis (LBD vs. APS) exhibited a significant predictive effect (P < 0.0001; ROC AUC = 0.84). Within the group of LBD patients, H&Y score showed a trend effect toward predicting responsiveness (P = 0.079; ROC AUC = 0.77), whereas the model was of no predictive value in APS patients (P = 0.75; ROC AUC = 0.63).
DISCUSSION
The present study was undertaken to evaluate the predictive value of 123I-iodobenzamide SPECT for dopaminergic responsiveness in patients with clinically suspected APS (the most clinically interesting patient group). The main finding of the present study is that 123I-iodobenzamide SPECT is not an independent predictor of dopaminergic responsiveness. In fact, the predictive value of D2R binding found in the present and earlier studies using univariate analyses can largely be attributed to the final clinical diagnosis and to other confounding variables such as patient age and H&Y score. In the present study, the determined degree of accuracy in predicting dopaminergic responsiveness across all patients (pooled LBD and APS groups: accuracy, 70%) is in line with earlier studies: Three larger studies using 123I-iodobenzamide SPECT to predict treatment response in de novo parkinsonism and in patients with questionable dopaminergic responsiveness (including mixed PD and APS populations) yielded a predictive accuracy of 80%–90% (12–14). A clinical follow-up study by the same group confirmed the predictive benefit of D2R imaging before treatment initiation (15). Although these earlier studies provided encouraging results, their limitations need to be noted: they did not account for interfering variables such as patient age, disease severity, and dopaminergic pretreatment before SPECT. Furthermore, rigorous criteria for clinical diagnoses were rarely applied. In contrast, a recent study investigated the relationship between D2R binding measured by 11C-raclopride PET and dopaminergic responsiveness in pretreated patients with parkinsonism. Although responders tended to show preserved D2R binding, no significant differences could be found between responders and nonresponders (22).
In the present study, sustained dopaminergic responsiveness was significantly associated not only with higher D2R binding but also with clinical diagnosis of LBD, low H&Y stages, and young age. Thus, it is questionable whether D2R binding is an independent predictor of dopaminergic responsiveness, given that the latter variables are known to influence striatal D2R binding. First, the clinical diagnosis of APS is linked per se to a decrease in D2R availability (15,16,23,24). The disease-specific progress of neurodegeneration leads to an early effect on basal ganglia and consecutive postsynaptic dysfunction in APS patients (25–27). Conversely, preserved postsynaptic dopaminergic neurotransmission in PD patients is typical (15). In fact, a compensatory upregulation of D2R is a consistent finding at early stages of the disease (28). Second, striatal BPND gained from 123I-iodobenzamide SPECT decreased with age, as described previously (21,29–31). This trend holds true both for healthy controls and for PD patients. Third, a downregulation of striatal D2R density in the clinical course of PD has been reported (16,32). Although a D2R upregulation is found at H&Y stages I and II, a decline toward control values commences with increasing disease severity (33). Nonetheless, it is still a matter of debate whether this association is a result of chronic dopaminergic therapy (34–36) or occurs independently of treatment and instead represents a structural correlate of disease progression (32). The available data on the impact of these variables in APS patients are sparse: in agreement with our results, Brooks et al. found no correlation between striatal binding of 11C-raclopride and disease duration in multiple-system atrophy or in progressive supranuclear palsy patients. Furthermore, dopaminergic pretreatment had no effect on striatal 11C-raclopride uptake (37). Of note, D2R binding in the present study tended to be higher in de novo LBD patients than in those without any preceding dopaminergic treatment. After correction for the above-mentioned confounders, there was no association between D2R binding and treatment response, either across all subjects or with regard to LBD and APS subgroups.
Finally, we preliminarily investigated which of the remaining variables are predictive of treatment response. Here, the final clinical diagnosis was the only significant predictor. The effect of aging on levodopa responsiveness has been investigated in PD patients before, whereby age at treatment initiation was inversely correlated with the magnitude of treatment response (38,39). Of note, the severity of levodopa-refractory symptoms (i.e., gait disturbance and postural instability) reportedly increases with age (40). In line with the notion that the aforementioned age-aggravated symptoms increase the H&Y stage, there was a significant association between age and H&Y stage in our PD patients (ρ = 0.68, P < 0.0001; not significant in APS). This finding may explain why only H&Y stage (and not age) reached a trend toward being a significant predictor of treatment response in the LBD group when both variables where entered simultaneously into the logistic regression model. Future studies with larger patient groups are clearly warranted to unravel this complex interplay.
CONCLUSION
Taken together, our findings underline the need to achieve the correct diagnosis at the earliest possible stage for accurate prediction of treatment response in parkinsonism. Given that the predictive effect of 123I-iodobenzamide SPECT is only indirect (via a diagnostic group effect, LBD vs. APS), the present study discourages the use of 123I-iodobenzamide SPECT for this purpose.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. This study was supported in part by GE Healthcare Buchler GmbH & Co. KG, Merz Pharmaceuticals GmbH, UCB Pharma, Medtronic GmbH, BMBF, the EU, the Danish Research Council, Bayer HealthCare AG, Philips Medical Systems, DFG, and Volkswagen Stiftung. No other potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank Sandra Dieni for her thoughtful comments on the manuscript.
Footnotes
Published online Oct. 10, 2013.
- © 2013 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication February 22, 2013.
- Accepted for publication June 4, 2013.