Abstract
Current pretargeting systems use noncovalent biologic interactions, which are prone to immunogenicity. We previously developed a novel approach based on the bioorthogonal reaction between a radiolabeled tetrazine and an antibody-conjugated trans-cyclooctene (TCO). However, the tumor-to-blood ratio was low due to reaction with freely circulating antibody-TCO. Methods: Here we developed 2 tetrazine-functionalized clearing agents that enable rapid reaction with and removal of a TCO-tagged antibody (CC49) from blood. Next, we incorporated this approach into an optimized pretargeting protocol in LS174T-bearing mice. Then we compared the pretargeted 177Lu-labeled tetrazine with 177Lu-labeled CC49. The biodistribution data were used for mouse and human dosimetry calculations. Results: The use of a clearing agent led to a doubling of the tetrazine tumor uptake and a 125-fold improvement of the tumor-to-blood ratio at 3 h after tetrazine injection. Mouse dosimetry suggested that this should allow for an 8-fold higher tumor dose than is possible with nonpretargeted radioimmunotherapy. Also, humans treated with CC49-TCO–pretargeted 177Lu-tetrazine would receive a dose to nontarget tissues 1 to 2 orders of magnitude lower than with directly labeled CC49. Conclusion: The in vivo performance of chemical pretargeting falls within the range of results obtained for the clinically validated pretargeting approaches in mice, with the advantage of potentially allowing for fractionated radiotherapy as a result of a lower likelihood of immunogenicity. These findings demonstrate that biologic pretargeting concepts can be translated to rapid bioorthogonal chemical approaches with retained potential.
The use of radiolabeled monoclonal antibodies (mAbs) for radioimmunotherapy of cancer is hampered by low tumor-to-nontumor ratios resulting in dose-limiting side effects in the bone marrow and in low tumor doses in patients (1). Pretargeting capitalizes on the tumor-seeking capabilities of long-circulating mAbs and on the fast distribution and clearance of small molecules with the aim of enabling the treatment of solid tumors in particular (2). Several pretargeting approaches have been tested in vivo (2–5), and to date, 2 have been evaluated in the clinic (2): systems that capture the probe using the extremely high affinity of the biotin–streptavidin interaction (association constant, ∼1015 M−1) and those that use antibody–hapten interactions (association constant, ∼108–1011 M−1). However, these biologic pretargeting systems, especially streptavidin constructs, are prone to immunogenicity, thus precluding repeated treatment cycles, which have proven critical to maximize the efficacy for other treatment modalities such as external-beam radiotherapy and chemotherapy. Furthermore, many of these systems require extensive reengineering and perturbation of the parent mAb.
Bioorthogonal chemical reactions (6) may be an alternative to biologic pretargeting interactions for the recruitment of the small radiolabeled probe to the tumor-bound mAb (Fig. 1). Such a chemical approach is less likely to give rise to immunogenicity and may in addition enable universal and straightforward tagging and in vivo tracking of mAbs without severe perturbation of the in vivo properties of the parent construct. Although the use of organic chemistry in living beings is a highly sought-after approach, only the inverse-electron-demand Diels–Alder reaction between strained trans-cyclooctene (TCO) and electron-deficient tetrazines has so far demonstrated sufficient potential for such demanding conditions (6–10). We reported previously that the use of this reaction could indeed be extended to a living system, showing pronounced and specific localization of an 111In-labeled DOTA-tetrazine probe in LS174T-tumored mice that were pretargeted with anti-TAG72 mAb CC49-TCO (8). However, the tumor-to-blood (T/B) ratio was still low because of the reaction between 111In-tetrazine and freely circulating CC49-TCO. Recently, the groups of Weissleder and Lewis achieved similar results with, respectively, an 18F- and 64Cu-labeled tetrazine (9,10). So far the inverse-electron-demand Diels–Alder approach has not led to an improvement of T/B ratio compared with directly labeled mAb. Ideally, the probe should be administered when the mAb has cleared completely from circulation. Since in humans intact mAbs take weeks to clear, requiring an unrealistic on-tumor stability of the mAb tag, they can instead be removed by injection of a clearing agent (CA). In the clinic, biotin-functionalized, liver-directed CAs have successfully been used to quickly capture streptavidin-tagged antibodies, boosting T/B ratios (11).
Tumor pretargeting with the inverse-electron-demand Diels–Alder reaction involving TCO-modified mAb and radiolabeled tetrazine.
To extend the concept of organic chemistry in mice to effective pretargeted imaging and radiotherapy with potential for clinical translation, we set out to match the performance of the clinically validated biologic pretargeting interactions with fast bioorthogonal organic chemistry. We developed a chemical clearing approach (compounds 3 and 4 in Fig. 2) that enables the controlled manipulation and removal of tagged biomolecules from blood, and we incorporated this into an optimized pretargeting protocol with a TCO with increased reactivity and stability (12), which led to a doubling of the tetrazine tumor uptake and a 125-fold improvement of the T/B ratio at 3 h after injection. We used a tetrazine labeled with 177Lu (half-life [t1/2], 6.7 d), which, in addition to γ, has a β emission that can be used for radiotherapy. We then demonstrated in a dosimetry study that the new protocol should allow for an 8-fold higher total tumor dose than is possible with nonpretargeted radioimmunotherapy, which is on a par with the noncovalent pretargeting approaches.
Pretargeting components: TCO-NHS (1) and DOTA-tetrazine (2) and schematic representations of galactose-albumin-tetrazine (3) and polystyrene (PS) beads coated with tetrazine-conjugated albumin (4).
MATERIALS AND METHODS
Syntheses of Pretargeting Components
CC49 production, TCO (1) synthesis, and conjugation and the synthesis of DOTA-tetrazine (2) (Fig. 2) have been described elsewhere (8,12). The syntheses of other compounds and more experimental details are reported in the supplemental data, which are available at http://jnm.snmjournals.org.
DOTA-Tetrazine Radiolabeling
DOTA-tetrazine (2) (2 mg/mL in 0.2 M ammonium acetate buffer, pH 7.0) was mixed with a suitable amount of 177LuCl3 or 111InCl3 (PerkinElmer) in 0.2 M ammonium acetate pH 5.5 and incubated at 60°C for 5 min. The labeling mixture was then mixed with 10 mM diethylenetriaminepentaacetic acid (5 μL) and a 20 mg/mL gentisic acid solution in saline (pH 6.5) and incubated for 5 min more. The radiochemical yield and purity were assessed by radio–instant thin-layer chromatography and radio–high-performance liquid chromatography, respectively, after which the labeling mixture was diluted with sterile saline for animal experiments. The specific activity of 177Lu-2 used for biodistribution and that of 111In-2 used for SPECT/CT imaging was 0.07–0.15 and approximately 6 MBq/nmol, respectively.
CC49-TCO Radiolabeling
Radioiodination of CC49-TCO was performed with the Bolton–Hunter method. Briefly, an adequate amount of Na125I (PerkinElmer) in 50 μL of phosphate-buffered saline was mixed with 1 μg of Bolton–Hunter reagent (SHPP; Pierce Protein Research) and 100 μg of chloramine-T. The solution was mixed for 10–20 s, 125I-SHPP was extracted into toluene, and the solution was evaporated to dryness under N2. CC49-TCO (0.1–0.5 mg in 50–250 μL of phosphate-buffered saline) was added to the 125I-SHPP, the pH was adjusted to 9 with 1 M Na2CO3, and the reaction mixture was incubated at room temperature for 30–60 min. After incubation, the labeling yield was determined by radio–instant thin-layer chromatography. The 125I-labeled mAb was purified twice (Zeba spin desalting columns [Pierce Protein Research], 40-kDa molecular weight cutoff), and the radiochemical purity of the 125I-CC49-TCO solution was determined by radio–instant thin-layer chromatography, size-exclusion high-performance liquid chromatography, and sodium dodecyl sulfate polyacrylamide gel electrophoresis. For animal experiments, the specific activity of the 125I-CC49-TCO was adjusted to 2–4 kBq/μg by adding nonradioactive CC49-TCO.
Animal Experiments
All experiments were performed on tumor-free or tumor-bearing nude female BALB/C mice (20- to 25-g body weight; Charles River Laboratories) according to the principles of laboratory animal care (13) and the Dutch national law “Wet op de Dierproeven” (Staatsblad 1985, 336). Mice were inoculated subcutaneously with 5 × 106 LS174T cells in 100 μL of sterile phosphate-buffered saline and were used 7–10 d later, when the tumors reached 70–200 mm3. The mice were euthanized by cervical dislocation, blood was withdrawn, and selected organs and tissues were harvested and blotted dry. All samples were weighed and then combined with 1 mL of phosphate-buffered saline. The sample radioactivity was counted in a γ counter (Wizard 1480; PerkinElmer) along with standards to determine the percentage injected dose (%ID) per gram and the %ID per organ. The tissues from dual-isotope experiments were measured using energy windows of 10–80 keV and 155–380 keV for 125I and 177Lu, respectively, with cross-contamination correction.
CC49-TCO Blood Clearance with CA Administration
Two groups of 3 tumor-free mice were injected with 125I-labeled CC49-TCO (average of 9 TCOs/mAb, 20 μg/100 μL per mouse; ∼0.2 MBq), and blood samples (∼20 μL) were withdrawn from the vena saphena after 5 and 30 min. Thirty-five minutes after mAb injection, each mouse received an intravenous injection of 3 (120 μg/100 μL per mouse) or 4 (4 × 107 beads/100 μL per mouse). More blood samples were withdrawn, and the animals were euthanized 210 min after mAb injection. One more group of 3 tumor-free mice was injected intravenously with 125I-labeled CC49-TCO (100 μg/100 μL per mouse, ∼0.4 MBq). The mice were serially bled at selected times and euthanized 4 d after mAb injection.
Pretargeting Protocol and SPECT Imaging
Two groups of 4 tumor-bearing mice were injected with 125I-labeled CC49-TCO (average of 7 TCOs/mAb, 100 μg/100 μL per mouse; ∼0.4 MBq) followed by 1 dose of 3 30 h after mAb injection or by 2 doses 30 and 48 h after mAb injection. Two hours after the (last) CA injection, the mice received 6.7 nmol of 177Lu-2 (8.52 μg/80 μL containing 100 μg of gentisic acid; ∼0.5 MBq) and were euthanized 3 h after tetrazine injection, after which organs and tissues of interest were counted for radioactivity.
For SPECT/CT imaging, a tumor-bearing mouse was injected with CC49-TCO, compound 3, and 111In-2 (6.7 nmol/80 μL; ∼42 MBq) following the optimized protocol. Approximately 90 min after tetrazine injection, the mouse was anesthetized with isoflurane and imaged on a small-animal SPECT/CT system (NanoSPECT/CT; Bioscan). Three hours after tetrazine injection, the mouse was euthanized by anesthesia overdose and a high-resolution SPECT/CT scan was obtained. The postmortem SPECT data were quantified by drawing volumes of interest in tumor, kidneys, liver, and thigh muscle using InVivoScope (Bioscan).
Dosimetry
Groups of 4 tumor-bearing mice were injected either with 177Lu-DOTA-CC49 (100 μg/100 μL per mouse; ∼0.5 MBq, supplemental data) or according to the optimized pretargeting protocol. One group of mice injected with 177Lu-DOTA-CC49 and one injected with pretargeted 177Lu-tetrazine 2 were used to evaluate the early blood distribution of the 2 tracers and were then euthanized 3 and 1 h after 177Lu-DOTA-CC49 and 177Lu-2, respectively. All other groups were euthanized at various times up to 7 d after tracer injection. Two additional groups of tumor-bearing mice were used for excretion profiles. Radiation dosimetry was estimated for a male human model (standard 73-kg adult) and for a voxelized mouse model using the biodistribution data according to the MIRD methodology. Activity concentration (%ID/g) in selected tissues was converted to %ID per organ and corrected for radioactive decay to the time of injection. The time–activity curves were fitted by a 2-phase exponential clearance function or by the trapeze method and integrated analytically to provide the organ residence times. The time–activity curve of the tumor was fitted by a monoexponential uptake function. The residence time of the red marrow was calculated as a fraction of the blood residence time (τmarrow = 0.08 τblood).
Radiation doses to the mouse organs were evaluated using the MIRD methodology, where the S factors (Starget←source) were calculated using a voxelized model of a mouse and the electron transport program EGSnrc (National Research Council Canada) to calculate energy deposition. The 4-dimensional digital mouse whole-body phantom of Segars et al. (14) was voxelized on a 0.2-mm grid and modified to include intestine wall and content, stomach wall and content, bladder wall and content, a 20-μL gallbladder, and a 0.53-g tumor (left side of the neck). Twenty-seven independent simulations, each for 107 decays, were performed with the program EGSnrc to calculate the energy deposition in each target organ for each source organ. The simulation included all decay particles from 177Lu (www.nndc.bnl.gov/MIRD). Human radiation dose estimates were calculated using the residence times of 177Lu-DOTA-CC49 and 177Lu-2 and the OLINDA/EXM program.
Data Analysis
The data are presented as mean %ID per gram or per organ ± 1 SD. Curve fitting and 2-tailed unpaired t tests were performed with Prism, version 4.1 (GraphPad). The difference between 2 data was considered statistically significant when the P value was less than 0.05. The error bars on the mouse and human radiation dose estimates are commensurate with the errors on the residence times (Supplemental Table 3) and range from 20% to 50%.
RESULTS
Component Development
The TAG-72–targeting mAb CC49 was reacted with a new TCO-N-hydroxysuccinimide (NHS) derivative with increased reactivity and in vivo stability (1, k2 = 2.7 ± 0.0 × 105 M−1s−1; 3.94-d stability half-life in vivo (12)), which afforded an average of 7–9 TCO groups per mAb. All radiolabeled mAb constructs were of greater than 98% purity.
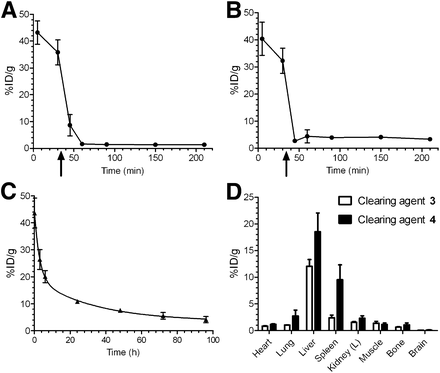
Both CAs were evaluated in vivo with respect to their mAb-TCO binding and clearing efficacy. In tumor-free mice, 125I-CC49-TCO (100 μg) without CA exhibited an expected slow blood clearance (t1/2,α = 2.0 h; t1/2,β = 23.5 h; Fig. 3C). To test whether the CAs would effectively remove a residual amount of circulating mAb several days after injection, a lower amount (20 μg) of 125I-CC49-TCO was injected followed by 3 or 4 after 35 min. Within 30 min, the 125I-CC49-TCO blood levels diminished, respectively, 22-fold (to 1.62 ± 0.35 %ID/g, Fig. 3A) and 12-fold (to 2.70 ± 0.40 %ID/g, Fig. 3B). However, for 4 a small increase in blood radioactivity to approximately 4 %ID/g was observed after the initial drop. No such rebound effect was detected in mice treated with 3. The subsequent biodistribution study (Fig. 3D) showed most of the radioactivity in the liver (12.05 ± 1.29 %ID/g) and minor uptake in the spleen (2.37 ± 0.53 %ID/g) of the mice treated with 3, and for 4 high uptake in both liver and spleen (18.55 ± 3.51 and 9.55 ± 2.77 %ID/g, respectively). On the basis of these results, CA 3 was chosen for further evaluation.
Blood clearance of 125I-CC49-TCO with and without use of CAs: 20 μg of mAb per mouse followed by 120 μg of galactose-albumin-tetrazine (3) (A), 20 μg of mAb followed by 4 × 107 beads coated with albumin-tetrazine (4) (arrows indicate time of CA injection) (B), 100 μg of mAb per mouse without CA (C), and biodistribution of 125I-CC49-TCO 210 min after mAb injection and after 3 or 4 (D). Data points represent mean %ID/g ± SD (n = 3).
Pretargeting Protocol Optimization and SPECT/CT Imaging
From a dosing study, we found 160 μg of 3 per mouse to give the optimal clearing efficacy (supplemental data). To further increase the T/B ratio, we then evaluated the effect of a single dose versus a double dose of 3 on the blood and tumor content of both pretargeting components, 125I-CC49-TCO and 177Lu-2, in a dual-isotope biodistribution experiment (Fig. 4). Tumor-bearing mice received intravenous injections of 1 or 2 CA doses (30 h, or 30 and 48 h after mAb injection) followed by 177Lu-2 2 h after the last dose and were euthanized 3 h later. A single CA dose already reduced the 125I-CC49-TCO in blood to 1.16 ± 0.43 %ID/g (Fig. 4A), compared with the 8.47 ± 4.12 %ID/g we previously had to contend with without CA. Also, only low levels of 125I radioactivity were detected in blood-rich tissues such as heart and lung. The radioactivity in the liver 5 h after CA injection (4.20 ± 1.16 %ID/g) was significantly lower than that found at 3 h in the initial biodistribution experiment (12.05 ± 1.29 %ID/g; Fig. 3D), supporting the rapid 3/125I-CC49-TCO metabolism and radioiodine elimination from the hepatocytes (15). To our satisfaction, a second dose of compound 3 further reduced the amount of 125I-CC49-TCO in blood (0.19 ± 0.04 %ID/g) and in all other considered organs, and the T/B, tumor-to-liver, and tumor-to-spleen ratios doubled or tripled (Supplemental Fig. 4A).
Dual-isotope biodistribution of 125I-CC49-TCO (A) and 177Lu-tetrazine 2 (B) in LS174T tumor–bearing mice treated with 1 dose (30 h after mAb injection, solid bars) or 2 doses (30 and 48 h after mAb injection, open bars) of galactose-albumin-tetrazine (3). Mice were injected with 177Lu-2 2 h after last CA and were euthanized 3 h later. Bars represent mean %ID/g ± SD (n = 4). *P < 0.05. **P < 0.005. ***P < 0.001.
The elimination of non–tumor-bound CC49-TCO translated into a greatly improved distribution of 177Lu-2 (Fig. 4B). We reduced the amount of tetrazine from the 17 nmol used in our previous studies without CA (8) to 6.7 nmol (1.2 molar equivalents with respect to TCO). As a result of this factor and the reduced CC49-TCO in circulation, tumor uptake increased from 3.12 ± 0.87 %ID/g to 7.45 ± 1.46 %ID/g and 6.13 ± 1.09 %ID/g with 1 and 2 CA doses, respectively. Importantly, the administration of a second CA dose significantly increased the tumor-to-nontumor ratios for blood (254 vs. 46), muscle (246 vs. 128), and other tissues (Supplemental Fig. 4B).
SPECT/CT imaging of a mouse injected with CC49-TCO followed by 2 clearing steps demonstrated a high 111In-tetrazine uptake in tumor and low retention in nontarget organs (Fig. 5 and Supplemental Fig. 5). At 3 h, the SPECT data showed tumor-to-kidney, tumor-to muscle, and tumor-to-liver ratios of 8, 190, and 55, respectively.
SPECT/CT image (postmortem, maximum intensity projection) of CC49-TCO pretargeted mouse bearing LS174T xenograft, 3 h after 111In-tetrazine 2 injection. SPECT/CT image of live mouse is available as Supplemental Figure 5.
Dosimetry
Mice bearing LS174T xenografts were administered either 177Lu-DOTA-CC49 or CC49-TCO pretargeted 177Lu-2 (using 2 clearing steps), and the 177Lu biodistribution was evaluated up to 1 wk after radioactivity injection. 177Lu-DOTA-CC49 exhibited a typical mAb biodistribution with a sustained circulation (t1/2,α = 1.0 h; t1/2,β = 32.6 h; Supplemental Fig. 6A) and a substantial radioactivity retention in blood-rich organs (Table 1 and Supplemental Table 1). As a consequence of the high levels in the blood, tumor uptake was also high, with 73.26 ± 26.69 %ID/g after 3 d. We also found activity in the mouse intestine due to hepatobiliary mAb elimination, but only 18.66 ± 2.26 %ID had been excreted 4 d after 177Lu-DOTA-CC49 injection.
Tumor-to-Organ Ratios for 177Lu-DOTA-CC49 in Mice Bearing LS174T Colon Carcinoma Xenografts
In contrast, in mice pretreated with CC49-TCO, 177Lu-2 cleared rapidly from the blood, with an 11-min half-life (Supplemental Fig. 6B) and all nonreacted probe was eliminated via the urine (93.75 ± 3.15 %ID 1 h after injection). After only 1 h, the tumor exhibited the highest 177Lu-2 uptake (4.71 ± 1.13 %ID/g) among the considered tissues, and tumor uptake peaked (5.38 ± 0.48 %ID/g) at 3 h whereas the remaining activity in blood, muscle, and bone decreased rapidly (Table 2 and Supplemental Table 2).
Tumor-to-Organ Ratios for CC49-TCO–Pretargeted 177Lu-Tetrazine 2 in Mice Bearing LS174T Colon Carcinoma Xenografts
These mouse biodistribution and excretion data were used to estimate the absorbed doses to tumor and normal organs in mice and to normal organs in humans. For both procedures, the dose-limiting organ in mice is the bone marrow. As expected, the estimated dose to the tumor in mice (Table 3) injected with directly labeled CC49 is high (9,573 mGy/MBq), but the bone marrow and the liver also receive high doses: respectively, 1,136 and 942 mGy/MBq. Although the estimated dose to the tumor in mice injected with pretargeted 177Lu-tetrazine is lower (479 mGy/MBq), much lower doses are delivered to the bone marrow (7 mGy/MBq) and to all other nontarget organs and tissues, including those of the urinary tract. In a standard adult man treated with pretargeted 177Lu-tetrazine, the estimated dose in most organs is 1–2 orders of magnitude lower than with 177Lu-DOTA-CC49 (Table 4). The primary and secondary critical organs for 177Lu-DOTA-CC49 are the liver (1.33 mGy/MBq) and ovaries (1.13 mGy/mBq), whereas those for pretargeted 177Lu-tetrazine are the bladder wall (0.104 mGy/MBq) and the kidneys (0.0425 mGy/MBq). The effective dose for intact mAb and pretargeted tetrazine is 0.447 and 0.0195 mSv/MBq, respectively.
Radiation Dose in Mouse Organs and Tissues in mGy/MBq
Organ Radiation Doses in Adult Men (73 kg) in mGy/MBq
DISCUSSION
We sought to develop a CA capable of eliminating circulating mAb-TCO to explore the full therapeutic potential of tumor pretargeting with the inverse-electron-demand Diels–Alder reaction. In pretargeting, CAs should rapidly bind the mAb in blood and remove it from circulation to the liver or spleen without blocking the tumor-bound mAb. Therefore, they should not diffuse out of the vascular compartment or produce tag-binding species on catabolism. We developed 2 types of agent that function through active or passive liver targeting (Fig. 2). For active liver targeting, we turned to galactose-mediated liver uptake via Ashwell receptors on hepatocytes, which has successfully been used in the clinic for biotin- and galactose-functionalized albumin and dendrimer CAs (11). We synthesized an albumin scaffold carrying tetrazine and galactose moieties (compound 3) for reaction with the mAb-bound TCO and interaction with the Ashwell receptors in liver, respectively. For passive liver targeting, we used polymer microparticles, because most intravenously injected particles clear via the reticuloendothelial system within minutes (16). In addition, the particle size precludes extravasation from the central vascular compartment and allows for high tetrazine loading. We prepared an albumin–tetrazine conjugate and used it to coat the surface of polystyrene beads (compound 4) to be used as a model particulate CA.
In a preliminary evaluation in tumor-free mice, both CAs were capable of removing 125I-CC49-TCO from the circulation (Figs. 3A and 3B), thus indicating that an efficient reaction occurred between the tetrazine groups on the CAs and the TCO on the mAb in blood. However, for 4 a small increase in blood radioactivity was observed after the initial drop, possibly caused by CC49-TCO reequilibration from physiologic compartments (17). This rebound was not due to 125I recirculation: at the time of mouse euthanasia the activity in stomachs and thyroids was low (1.66 ± 0.70 and 0.59 ± 0.15 %ID/organ), confirming in vivo stability of the iodinated mAb. No such rebound effect was detected in mice treated with 3, possibly because, on reequilibration, galactosylated albumin is still present in the circulation to some extent whereas the even more quickly clearing microparticles are not (18,19). The subsequent biodistribution study (Fig. 3D) confirmed the clearing mechanisms of both agents, with most of the radioactivity in the mice treated with 3 found in the liver whereas for 4 a high uptake was found in both liver and spleen, the main organs of the reticuloendothelial system. From the radioactivity in the intestine (22.83 ± 4.92 %ID/organ with 3 vs. 12.97 ± 1.82 %ID/organ with 4), it appears that mice treated with 3 excreted radioactivity more quickly than those that received 4. On the basis of this preliminary evaluation, we chose to include 3 in an optimized pretargeting protocol.
After determining the optimal dose of CA 3, we continued with a comparison between a single and double CA dose in a dual-isotope biodistribution experiment (Fig. 4). The increased stability of the new TCO (12) allowed for a longer interval between mAb and probe administration than was possible with our previous experiments. We also reduced the amount of injected tetrazine 2 (from 17 to 6.7 nmol/mouse, that is, 1.2 equivalents with respect to TCO) to increase the radioactivity %ID/g in tumor. As a result of the reduction in TCO blood concentration from about 0.4 μM when not using a CA to 50 and 10 nM with 1 and 2 doses of CA 3, respectively, the 177Lu-2 retention in blood at 3 h after injection was extremely low (respectively, 0.17 ± 0.06 and 0.03 ± 0.01 %ID/g) and accordingly the T/B ratio increased exponentially, from about 2 without a CA to 80 with 1 CA dose to 250 with 2 doses (Supplemental Fig. 4). This 125-fold improvement in T/B ratio, and the biodistribution profile in general are within the range of values achieved in mice with the established, biologic, pretargeting methods (20–23).
These encouraging results prompted us to conduct a dosimetry comparison between direct radioimmunotherapy with 177Lu-DOTA-CC49 and our optimized chemical pretargeting approach. The typical mAb biodistribution data obtained for 177Lu-DOTA-CC49, with a sustained circulation, high tumor uptake, and substantial radioactivity retention in blood-rich organs and in excretory organs, were in good agreement with those previously published for other 177Lu-CC49 constructs (24). In contrast, in mice pretreated with CC49-TCO, 177Lu-2 cleared rapidly from blood and all nonreacted probe was eliminated via the urine within a few hours. The apparent decrease in tumor values after the peak 177Lu-2 uptake in tumor 3 h after injection (5.38 ± 0.48 %ID/g) is a consequence of radioactivity dilution due to tumor growth from approximately 0.4 to 1.5 g in 7 d. The absolute amount of 177Lu in the whole tumor (2.16 ± 0.43 %ID 3 h after 177Lu-2 injection) did not decrease significantly with time, because 1.49 ± 0.56 %ID was still present in the tumor 7 d later. This is a significant difference when compared with results obtained with CC49-scFv-streptavidin fusion protein followed by 177Lu-DOTA-biotin (25,26). For instance, Lewis et al. (26) observed a 10-fold decrease in 177Lu %ID/g in LS174T xenografts over 1 wk, unrelated to tumor growth. Our findings suggest strong binding of the CC49-TCO to TAG-72 in the tumor and in vivo stability of the inverse-electron-demand Diels–Alder reaction product between the tumor-bound TCO and the tetrazine, which are essential features for the delivery of a therapeutic dose.
Besides the tumor, the kidney was the only organ to retain the 177Lu-2, albeit to a lesser extent (1.58 ± 0.14 %ID/g at 3 h) and less than previously found for 177Lu- and 90Y-labeled biotin (20,26). As a result of the good tumor uptake and fast clearance from blood in the pretargeted mice, the T/B ratio was approximately 200 already at 3 h after 177Lu-2 injection and increased to more than 500 by the end of the experiment (Table 2). In contrast, the T/B ratio for 177Lu-DOTA-CC49 exceeded 1 only 1 d after mAb injection and 3 d later peaked at about 30 (Table 1). The rapid and selective reaction of the tetrazine with the chemically tagged tumors, and the resulting excellent tumor-to-nontumor ratios, were also demonstrated in a corresponding SPECT/CT imaging study of live mice using 111In-2 (Fig. 5 and Supplemental Fig. 5).
The estimated radiation doses to the mouse organs (Table 3) were derived from the biodistribution data using the MIRD methodology. The dose-limiting organ for both 177Lu-CC49 and pretargeted 177Lu-tetrazine was the bone marrow. On the basis of Table 3 and the maximum tolerated dose of 2.5 Gy for marrow (27), we estimated that less than 21 Gy would be delivered to the tumor with 177Lu-CC49 whereas the pretargeting approach would deliver 8-fold more, 171 Gy, exceeding 80–100 Gy, commonly considered the threshold for clinical efficacy for radioimmunotherapy of solid tumors (28). Human dosimetry indicated that, similar to mice, humans treated with pretargeted 177Lu-2 would receive a dose to nontarget tissues 1 to 2 orders of magnitude lower than with 177Lu-CC49 (Table 4). Our results suggest that chemical pretargeting can deliver to the tumor a therapeutic radiation dose up to 1 order of magnitude higher than nonpretarged radioimmunotherapy, which is on a par with the results from biologic pretargeting studies in mice (20,21,23,29).
CONCLUSION
The use of a rapid bioorthogonal clearing approach and an optimized chemical pretargeting protocol, combined with a TCO with increased stability and a high reactivity of k2 = 2.7 ± 0.0 × 105 M−1s−1, resulted in a selective and effective on-tumor reaction with, and retention of, a small and quickly clearing radiolabeled probe and high tumor-to-nontumor ratios. The predicted 8-fold higher total tumor dose of this approach compared with conventional radioimmunotherapy is similar to results obtained in mice with the clinically validated, noncovalent pretargeting approaches, which have association rates ranging from 5 × 105 to 7.5 × 107 M−1s−1. The chemical components are less likely to give rise to immunogenicity, potentially enabling repeated treatment cycles and therefore increased anticancer efficacy. The protein modification is straightforward with minimal perturbation of the tagged construct and can be applied to mAbs (fragments), other biologics, and nanoparticles. We expect that this technology will also find application as a companion imaging tool in biologics development and, specifically for the CA, in the controlled removal of, for example, antibody–drug conjugates or radiolabeled antibodies from blood. Finally, the presented components may allow organic chemistry to be conducted in humans.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. No potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank Ebo Bos and Frans Kaspersen (former Organon) for insightful discussions, and we thank Ron Versteegen (SyMO-Chem), Sidney Wessels, Iris Verel, Monique Berben (Philips Research), Caren van Kammen, Carlijn van Helvert, and Melanie Blonk (Maastricht University) for support with chemical and in vivo experiments.
Footnotes
Published online Oct. 3, 2013.
- © 2013 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication March 26, 2013.
- Accepted for publication July 3, 2013.