Abstract
Previous imaging studies have suggested that there is an age-related decline in brain serotonin (5-hydroxytryptamine) measures in healthy subjects. This paper addresses whether the availability of 5-hydroxytryptamine receptor 1B (5-HT1B) is seen to decrease with aging via PET imaging. Methods: Forty-eight healthy control subjects (mean age ± SD, 30 ± 10 y; age range, 18–61 y; 33 men, 15 women) underwent 11C-P943 scanning on a high-resolution PET tomograph. Regions were examined with and without gray matter masking, the latter in an attempt to control for age-related gray matter atrophy on nondisplaceable binding potential (BPND) as determined by a validated multilinear reference tissue model. Results: 5-HT1B BPND decreased in the cortex at an average rate of 8% per decade without and 9% with gray matter masking. A negative association with age was also observed in all individual cortical regions. Differences in the putamen and pallidum (positive association) were significant after adjustment for multiple comparisons. No sex- or race-related effects on 5-HT1B BPND were found in any regions. Conclusion: These findings indicate that age is a relevant factor for 5-HT1B in the cortex of healthy adults.
Serotonin (5-hydroxytryptamine) has been implicated in the aging of the brain. Preclinical and clinical work has shown alterations in the functional and structural capacities of 5-hydroxytryptamine during aging (1,2). In translational neuroimaging, decreases with age have been found for the serotonin transporter, the 5-hydroxytryptamine receptors 1A and 2A (5-HT1A and 5-HT2A, respectively), and for cerebral glucose metabolism after administration of a selective serotonin reuptake inhibitor in many cortical areas (3–8).
Recently, evidence that aging has an effect on the 5-hydroxytryptamine system has also brought about a focus on 5-HT1B (1,9). 5-HT1B is part of the 5-HT1 family and is a G-protein–coupled metabotropic receptor spanning 7 transmembranes (10). 5-HT1B is expressed as an autoreceptor on serotoninergic neurons, where it is the predominant presynaptic modulator of 5-hydroxytryptamine release in the brain, and as a heteroreceptor on nonserotoninergic neurons (10). Although the only clinically available 5-HT1B medications are for headaches (the triptan class of 5-HT1B/D agonists), these receptors have become increasingly important in neurophysiologic functions and for behaviors as diverse as locomotor activity, drug abuse reinforcement (11), depression (12), and posttraumatic stress disorder (13), as well as learning, memory, and aggressive behavior (10).
Basic research in aging has shown large decreases (of ≤27%) in the density of 5-HT1B (5-HT1A apparently did not decrease) in elderly rats (9,14) and postsynaptic 5-HT1B messenger RNA in several brain regions (14), raising the possibility that aged animals may have relatively specific reduced 5-HT1B function. In addition, another study focused on 5-HT1B and aging and demonstrated that 5-HT1B knockout mice had an early age-related motor decline and decreased lifespan, thus increasing the plausibility that 5-HT1B is an important component of normal aging (1). An interesting finding of that paper was a comparative phylogenetic conservation of a genetic age effect from mice to humans suggesting that 5-HT1B may be a modulator of aging across species (1). To date, however, possible effects of aging on 5-HT1B have not, to our knowledge, been studied in healthy humans. The aim of this study was to investigate age effects in the brain of healthy subjects via the highly selective 5-HT1B ligand 11C-P943 using PET imaging.
MATERIALS AND METHODS
Subjects
Forty-eight healthy, medication-free volunteers were recruited through public advertisement to participate in the study. Subjects were between 18 and 61 y old (mean ± SD, 30 ± 10 y). Thirty-three (69%) were male. Thirty-seven (77%) were Caucasian, 5 (10%) were African-American, and 2 each were Hispanic, Asian-American, or multiracial. One subject (26 y old) was excluded on statistical grounds (>3 SDs above the mean cortical nondisplaceable binding potential [BPND]).
All subjects had a comprehensive screening assessment that included a complete physical examination with medical history, routine blood tests, pregnancy test, urine toxicology, and electrocardiography and a psychiatric evaluation with application of the structured clinical interview for diagnosis of axis I disorders from the Diagnostic and Statistical Manual of Mental Disorders, fourth edition. Individuals were excluded if they reported a diagnosis of current or lifetime axis I or II psychiatric disorders; current or past serious medical or neurologic illness (including a history of head injury with loss of consciousness); current pregnancy (as documented by pregnancy testing at screening and on the day of the PET study) or breast feeding; general MRI exclusion criteria; and significant alcohol or illicit substance abuse or dependence in the past 3 mo. All subjects were medication-free for a minimum of 6 wk at the time of the scan.
The study was performed under protocols approved by the Yale Human Investigation Committee, the Human Subjects Subcommittee of the Veterans Affairs Connecticut Healthcare System, the Yale University Radiation Safety Committee, the Yale–New Haven Hospital Radiation Safety Committee, and the Yale MRI Safety Committee. Subjects were recruited from New Haven and surrounding areas by advertisement, word of mouth, and referrals. Written informed consent was obtained from all participants after a full explanation of study procedures.
Radiochemistry
11C-P943 (R-1-[4-(2-methoxy-isopropyl)-phenyl]-3-[2-(4-methyl-piperazin-1-yl)benzyl]-pyrrolidin-2-one) was prepared as previously described (15) by N-methylation of the precursor with 11C-methyl triflate, using the PETtrace cyclotron and a TRACERLab FxC automated synthesizer (GE Healthcare). The GE Microlab was used in some of the preparations as a source of the requisite 11C-methyl iodide.
Scanning and Imaging Procedures
PET was performed with the selective 5-HT1B antagonist radiotracer 11C-P943. All scanning was performed on a high-resolution research tomograph (Siemens/CTI), which acquired 207 slices (1.2-mm slice separation) with a reconstructed image resolution of about 3 mm. A transmission scan with a 137Cs point source was obtained before the emission scan. The PET scans were acquired for 120 min at rest with a single intravenous injection of 630 ± 132 MBq and a high specific activity of 161 ± 71 MBq/nmol.
Structural MR images were obtained on a 3-T Trio system (Siemens Medical Solutions) with a circularly polarized head coil for each subject to exclude individuals with anatomic abnormalities and for coregistration. The dimension and voxel size of MR images were 256 × 256 × 176 and 0.98 × 0.98 × 1.0 mm, respectively.
Dynamic PET scan data were reconstructed with all corrections (attenuation, normalization, scatter, randoms, dead time, and motion), using the MOLAR algorithm (16) with the following frame timing: 6 × 30 s, 3 × 1 min, 2 × 2 min, and 22 × 5 min. Motion was additionally corrected by image smoothing with a gaussian filter of 3 mm in full width at half maximum and either by using an optical detector (Vicra; NDI Systems) or by coregistering each frame image to an early summed image (0–10 min after injection) with a 6-parameter mutual information algorithm (FLIRT, FSL 3.2; Analysis Group, FMRIB). Both motion corrections were added as a covariate in further analysis, and no significant differences were found in any of the regions studied (F1,45 = 0.01, P = 0.9144). As in previous P943 studies, PET data were used to produce a time–activity curve for the cerebellum, which was used as a reference. The multilinear reference tissue model has been previously validated in a P943 study and was used to produce parametric images of BPND (17).
A second summed image (0–10 min after injection) was created from the motion-corrected PET data and registered to the subject’s MR image, which in turn was registered (12-parameter affine transformation) to an MRI template (Montreal Neurologic Institute space).
Regions of interest were based on the Anatomic Automatic Labeling template delineated on an MRI template (18). The primary region was the cerebral cortex, which was a summed result of the frontal, occipital, parietal, and temporal cortices. A secondary analysis examined subcortical areas (amygdala, caudate, hippocampus, hypothalamus, pallidum, putamen, and thalamus). These results were obtained using individual parametric images that were resliced in template space using the PET-to-MRI and the MRI-to-template transforms.
To account for possible partial-volume effects, a binary gray matter mask (GMM) was used. For these results, individual MR images were segmented with FAST (FMRIB’s Automated Segmentation Tool, version 3.1) to obtain masking of gray matter, white matter, and cerebrospinal fluid. The individual GMMs were then applied to the regions from the Anatomic Automatic Labeling template to obtain the mean regional values limited to gray matter voxels.
Statistical Analysis
All outcomes were summarized descriptively and assessed for normality before analysis using normal probability plots and Kolmogorov test statistics. All outcomes were approximately normal. Correlations between age and 5-HT1B were calculated separately within, and averaged across, each region. A linear mixed model was used to model the independent and joint effects of age (continuous) and cortical region (within-subject factor) on BPND values. The interaction between region and age was modeled, and slopes for each cortical region were estimated post hoc. Within-subject correlations were accounted for by fitting 3 variance–covariance structures to the data (unstructured, compound symmetry, and heterogeneous compound symmetry) and then selecting the best-fitting structure according to the Bayesian information criterion. Subcortical regions were analyzed as secondary subregions and adjusted using the Bonferroni adjustment. All analyses were conducted using SAS, version 9.1 (SAS Institute Inc.).
RESULTS
Table 1 presents average 5-HT1B (±SD) BPND levels for cortical and segmented cortical gray matter regions, along with the slope and correlations with age in the 47 reported subjects. Significant negative associations between age and 5-HT1B BPND were observed in all cortical regions, and total cortical BPND averaged an 8% decline per decade studied (9% in total cortical gray matter using GMM).
Regional Mean BPND, Correlations Between Age and 11C-P943 BPND, and Slope for Cortical Regions of Interest
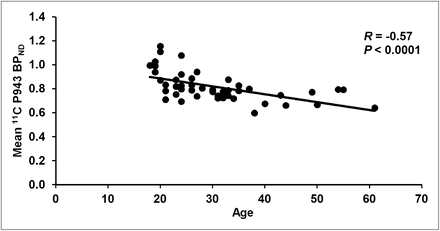
The results of the primary mixed model showed an overall significant effect of age on both total BPND (F1,45 = 21.6, P < 0.0001) and total BPND after GMM (F1,45 = 28.6, P < 0.0001), averaged across all cortical regions. These effects are depicted in Figures 1 and 2, respectively. Consistent with the univariate results, primary mixed-model slope estimates for the individual 5-HT1B BPND cortical regions (with and without GMM) remained significant (P = <0.01) with age (Figs. 3 and 4, respectively).
Mean cortical BPND for each subject along with age (in years).
Mean cortical BPND (after GMM) for each subject along with age (in years).
Mean BPND for each cortical region along with age (in years).
Mean BPND (after GMM) for each cortical region along with age (in years).
Subcortical region (without GMM) correlations were largely insignificant when age and 5-HT1B BPND were examined, with the exception of the hypothalamus (r = −0.28, P = 0.05), pallidum (r = +0.39, P = 0.01), and putamen (r = +0.41, P < 0.01). GMM subcortical results found a difference in the putamen (r = +0.32, P = 0.03) and a trend-level difference in the pallidum (r = +0.26, P = 0.08), with all other regions nonsignificant. Subcortical results remained significant after a multiple-comparison correction in the pallidum (Bonferroni-adjusted P = 0.05) and putamen (Bonferroni-adjusted P = 0.03) without GMM.
Sex and race had no significant effects on these results.
DISCUSSION
This work has furthered our understanding of the role of aging on the serotonin system by demonstrating age effects on 5-HT1B with the PET radiotracer 11C-P943. A decrease in BPND was found throughout cortical areas with increased age. These findings are consistent with the existing literature on aging and serotonin, which has shown decreases in the density of 5-HT1A and 5-HT2A in aging (3–6,8,19).
Different methods to control for cerebral atrophy were used in these cited studies. The current work used a gray matter segmentation to create a gray matter binary mask, and although this method is not necessarily sensitive to all partial-volume effects, the retention of findings argues against the possibility that cerebral atrophy is responsible for the decreases in 5-HT1B with aging. Furthermore, a recent study that examined 5-HT2A and aging found that a partial-volume correction was not necessary when similar parameters (cortical regions of interest, high-resolution scanning, and a healthy population) were examined (19).
Our sample comprised mostly male subjects (69%) who were younger than 61 y, and the sample was also limited in distribution across age regions (with most subjects under 40 y). This is a limitation to the current analysis, as it is susceptible to bias induced by fewer older subjects and sex (although no current sex effects were found). Further studies including a more balanced distribution of older subjects could be justified to verify that 5-HT1B decline is a linear phenomenon in advanced age. Although the multilinear reference tissue model is a validated method to examine these data, another potential limitation in this study was the lack of available arterial blood flow data in most subjects to rule out potential confounders such as age-related effects of the cerebellar time–activity curve.
The preliminary finding of increased 5-HT1B BPND in the pallidum and putamen (which retained significance after multiple corrections) could be of interest, as there are no current reports of increasing serotonin receptor brain levels with age in any region. If confirmed in further work, these subcortical areas could be a focus of a potentially novel process.
Finally, whereas the focus of this paper was to investigate age effects on 5-HT1B, the functional impact of this finding could extend into cognitive effects such as memory or learning that have been implicated in 5-HT1B decline in a host of preclinical work (1,9,14). In the only other work to date on an aged human population, postmortem examination found reduced 5-HT1B density in Alzheimer patients, compared with controls, in the frontal and temporal cortices (20). Interestingly, cognitive decline was associated with more 5-HT1B in that study. The authors attributed this finding to a compensation of 5-HT1B heteroreceptors for a deteriorated cholinergic system (i.e., the heteroreceptors, which function to inhibit acetylcholine, led to worse cognitive performance due to decreased acetylcholine in an already acetylcholine-deficient state in Alzheimer disease). It remains to be seen if 5-HT1B medications could have a clinical effect on age-specific cognitive problems, but the current results support further investigation of this potential learning and memory treatment.
CONCLUSION
The findings of this study indicate that age is a relevant factor for 5-HT1B in the cortex of healthy adults.
DISCLOSURE STATEMENT
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Both the P943 standard and the N-desmethyl precursor were provided by Pfizer, Inc. (Groton, CT). This project was supported by the National Institutes of Health through the following awards: R21 MH081103 (ARRA), R21 MH085627, and R21 AA018329. This project was also supported in part by the Research Council of Norway, Division for Science. Zubin Bhagwagar is a full-time employee of Bristol-Meyers Squibb. No other potential conflict of interest relevant to this article was reported.
Footnotes
Published online Jul. 31, 2012.
- © 2012 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication January 25, 2012.
- Accepted for publication May 9, 2012.