Abstract
One of the central unanswered questions in prostate cancer research is the significance of tyrosine kinase inhibitor (TKI)–induced improvements in 99mTc-methylene diphosphonate (99mTc-MDP) bone scans. Multitargeted tyrosine kinase inhibition has recently shown promise in the management of castration-resistant prostate cancer. In some cases, TKI inhibition has produced unprecedented improvements in bone metastases as detected by 99mTc-MDP bone scans. The significance of these improvements is not known. In order to gain insight about the effects of TKIs on bone scans in prostate cancer, we systematically evaluated images from a phase II study of sunitinib, a multitargeted TKI. Methods: We analyzed images and data from a previously reported open-label phase II study that enrolled 34 men with advanced castration-resistant prostate cancer. Participants received sunitinib in 6-wk cycles (50 mg daily; 4 wk on, 2 wk off). We examined baseline and 12-wk bone scan images. Partial response was defined as an improvement of at least 50% in previous metastatic lesions subjectively or a change from prior diffuse skeletal metastases (superscan) to recognizable individual metastatic lesions. Our primary objective was to define the incidence of at least partial bone scan response. We also examined concomitant changes in CT and prostate-specific antigen (PSA) evidence of disease. Results: Analysis at 12 wk revealed 1 partial response by the response evaluation criteria in solid tumors (RECIST) and 2 confirmed PSA responses. There were 25 subjects who underwent bone scans at both time points (baseline and week 12) and who had bone metastases detectable at baseline. Within that group of 25, we found 5 bone scan partial responses and 1 complete response. None of those 6 subjects exhibited a PSA response (≥50% decline from baseline) or RECIST response. Conclusion: We found a relatively high rate of 99mTc-MDP bone scan response to sunitinib among men with metastatic prostate cancer. Further, we found that none of the subjects exhibiting bone scan responses experienced concordant improvements in PSA or CT evidence of disease by accepted criteria. This discordance argues that osteoblastic assessment provides an incomplete assessment of treatment-induced changes. Rational development of multitargeted TKIs for prostate cancer requires improved understanding of treatment-induced bone scan changes. Optimal imaging strategies may include evaluation of perfusion or direct tumor activity.
Skeletal scintigraphy has long been a cornerstone of disease assessment in prostate cancer. 99mTc-methylene diphosphonate (99mTc-MDP) bone scans are widely used and provide an indirect measure of tumor activity because they detect tracer deposition by osteoblasts along bone mineralization fronts (1,2). Prostate cancer bone metastases can be imaged this way because they are associated with elevated activity by both osteoblasts and osteoclasts (3,4). Further, 99mTc-MDP bone scans are an established component of disease assessment in prostate cancer clinical trials (5).
Multitargeted tyrosine kinase inhibition with orally administered small-molecule tyrosine kinase inhibitors (TKIs) is an established strategy for the management of numerous malignancies including chronic myeloid leukemia, breast cancer, renal cell carcinoma, non–small cell lung cancer, melanoma, and others. It has only recently emerged as a potential treatment strategy for advanced prostate cancer. In particular, TKI therapy has preliminarily been shown to produce unprecedented improvements in the 99mTc-MDP bone scans of men with castration-resistant prostate cancer (CRPC) metastatic to bone (6). The clinical significance and mechanism responsible for these bone scan improvements have not yet been well defined.
There are several possible explanations for the dramatic bone scan changes. Treatment-induced bone scan changes may be caused by the death of tumor cells, changes in tumor perfusion, changes in peritumoral osteoblast activity, or other factors. Although no TKI has been approved for the management of prostate cancer, several agents have been examined in clinical trials. Formal evaluation of bone scan responses to each of these TKIs may provide additional insights.
Sunitinib is an orally administered TKI that inhibits several kinases including vascular endothelial growth factor receptor 2 (VEGFR2), platelet-derived growth factor receptor-β, and KIT. Sunitinib treatment of metastatic CRPC was examined in a randomized placebo-controlled phase III study. In that study, sunitinib improved overall response rate and progression-free survival but failed to demonstrate improvement in its primary endpoint, overall survival (7). Therefore, it is no longer in development for the treatment of prostate cancer. However, we previously observed instances of marked bone scan improvements among phase II study participants with metastatic CRPC treated with sunitinib at our institution.
To gain insight about the effects of multitargeted TKI therapy on bone scans in advanced prostate cancer, we analyzed data from that open-label phase II study of sunitinib for metastatic CRPC. Specifically, we examined changes in bone scan findings from baseline to the first repeated bone scan during treatment (12 wk). Our goals were to assess the frequency of improvement in bone scan assessment of disease and to demonstrate the presence or absence of concordance between bone scans and other accepted measures of disease activity (PSA and CT scans).
MATERIALS AND METHODS
We analyzed data from a previously described (8) open-label phase II study of sunitinib treatment of men with advanced CRPC. That study enrolled 34 eligible men with histologically confirmed adenocarcinoma of the prostate and evidence of progression despite castrate testosterone (serum testosterone < 50 ng/dL). Progression was defined as a rising PSA in 2 consecutive measurements at least 1 wk apart; PSA was required to be at least 2 ng/mL above the nadir value. Among those enrolled, 17 had received prior docetaxel chemotherapy. Concurrent bisphosphonate treatment was allowed. All participants provided written informed consent, and the study was approved by the Dana Farber/Harvard Cancer Center Institutional Review Board.
The primary endpoint of the trial was PSA response rate, defined as a confirmed PSA decline of at least 50% from baseline. A secondary endpoint was an objective response rate according to the response evaluation criteria in solid tumors (RECIST). All men were treated with sunitinib in 6-wk cycles consisting of 50 mg daily for 4 wk, followed by 2 wk off. Dose reductions to 37.5 or 25 mg were allowed. Treatment continued until intolerance to therapy or disease progression, defined as the presence of a new metastasis or a PSA increase of 25% or more above the nadir.
Serum PSA was measured on day 1 of each 6-wk cycle. Radiographic assessments were done at baseline, every 12 wk, and at study end or subject withdrawal. Bone scans were performed per institutional standard clinical practice using 740 MBq (20 mCi) of 99mTc-MDP, with anterior and posterior planar whole-body imaging performed at least 2.5 h after tracer injection; additional planar spot views were obtained as needed for clarification of ambiguous findings. Assessments took place during the scheduled 2-wk-off-treatment interval at the conclusion of the second cycle of study-directed therapy. Responses were assessed using RECIST. Landmark analysis was performed at week 12 to conform to initial reassessment guidelines as recommended by Prostate Cancer Clinical Trials Working Group II (5).
For the present analysis, we examined baseline and 12-wk bone scan images among study participants who had bone metastases at baseline and for whom those 2 imaging studies were completed. Two radiologists who were specialized in nuclear medicine, and 1 nuclear medicine physician, assessed each set of images in consensus. Bone scan changes were assessed according to the categories detailed in Table 1. Our primary objective was to define the incidence of at least a partial bone scan response during the interval between baseline and the 12-wk bone scan. We also examined concomitant changes in CT and PSA evidence of disease.
Bone Interpretation Criteria, Modified from Recommendations of Prostate Cancer Clinical Trials Working Group 2 (5)
RESULTS
Thirty-four eligible participants were enrolled in the study. A total of 17 had received docetaxel chemotherapy (median number of cycles, 8; range, 3–14 cycles). At baseline, 27 had detectable bone metastases. Bisphosphonates had been used in 17 subjects. The median duration of sunitinib treatment was 2 cycles (range, 1–15 cycles). The most common reason for discontinuation of therapy was PSA progression.
As previously reported (8), analysis at 12 wk revealed 1 partial response by RECIST. An additional 18 subjects had stable disease by RECIST at that time point. One confirmed PSA response was observed in each of the 2 groups (i.e., no prior docetaxel [group A] and docetaxel-resistant [group B]). An additional 8 men in group A and 7 men in group B had stable PSA at week 12.
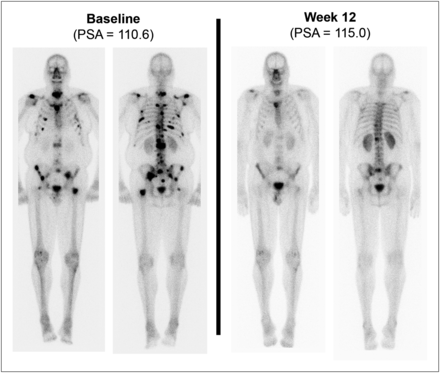
For the present analysis, 28 of the 34 enrolled subjects were assessed by bone scanning at baseline and the 12-wk follow-up (i.e., 6 subjects discontinued trial participation before the start of therapy or during the first 12 wk of therapy). None of those subjects had started bisphosphonate therapy between the baseline and follow-up bone scans. Within that group of 28, 3 did not have bone metastases at baseline and were therefore not evaluable for bone scan response. Among those 25 subjects who had bone scans at both time points and bone metastases detectable at baseline, we found 6 cases of at least partial bone scan response (partial response plus complete response; Table 2). Bone scan images and clinical data relevant to 3 of these 6 cases are summarized in Figures 1–3⇓⇓ (the other 3 cases are described in Supplemental Figs. 1–3; supplemental materials are available online only at http://jnm.snmjournals.org). None of those 6 subjects exhibited a response by accepted PSA criteria (≥50% decline from baseline) or RECIST.
PSA and Radiologic Response of All Patients as Grouped by Bone Scan Response
Complete bone scan response, with no lesion to indicate metastatic disease on follow-up scan. CT revealed stability of retroperitoneal nodal metastasis. PSA declined by 35%.
Interval resolution or markedly decreased intensity of multiple bone metastases involving spine, sternum, multiple bilateral ribs, scapulae, pelvic bones, and both femora, categorized as partial response. PSA increased by 4%.
Interval resolution or marked improvement of all previously seen bone lesions, categorized as partial response. New liver metastasis was found on CT. PSA increased by 252%.
To qualitatively assess concordance between bone scan and PSA assessment of response, we plotted the interval percentage change in PSA for subjects grouped by bone scan response category (Fig. 4). Qualitatively, bone scan response category correlated poorly with percentage change in PSA.
PSA changes among subjects grouped by bone scan response. Subjects grouped by bone scan response category (n = 25) display variable PSA changes during same study treatment period. CR = complete response; NE = not evaluable; PD = partial disease; PR = partial response; SD = stable disease.
DISCUSSION
We analyzed baseline and week-12 bone scan images of men in a phase II study of sunitinib for metastatic CRPC. Among the 25 subjects who could have exhibited a response, we found 6 cases of responses by bone scan. None of those 6 subjects exhibited a response at that time point by PSA criteria or by RECIST. The observed incidence of bone scan improvement is surprising given that CT and PSA responses were uncommon. These findings may be relevant to the assessment of therapeutic response to other multitargeted TKIs.
The reported assessments may underestimate sunitinib-induced bone scan effects. Follow-up imaging and PSA assessments took place during the 2-wk scheduled off-treatment interval at the conclusion of the second cycle of therapy (6-wk cycle: 4 wk on, 2 wk off). This treatment schedule may have led to prescan regression of improvements that occurred during the 4-wk on treatment. As most participants were removed from the study because of PSA progression that rose to 25% above its nadir, the duration of bone scan improvements is not known.
The assessment of therapeutic response in clinical trials is a topic of much discussion. Metastatic CRPC commonly features bone metastases (80%–90% in recent phase III trials (9,10)), often as the only site of metastasis. RECIST does not adequately address this. The Prostate Cancer Clinical Trials Working Group 2 recommends independent reporting of PSA, imaging, and clinical measures. It recommends assessment of bone scans only for the presence or absence of 2 or more new lesions, compared with a prior scan (5). Prostate cancer treatment trials have not historically been designed to systematically assess for bone scan responses that are discordant from other measurements of activity.
The relatively high incidence of bone scan improvement observed within this analysis is important in light of the promising early-phase activity demonstrated by cabozantinib (XL184), a TKI with targets that overlap those of sunitinib. Available data from the early clinical experience with cabozantinib reveal a high incidence of bone scan improvements but low rates of response by PSA criteria or RECIST (6). Despite a growing number of therapies that improve survival among men with CRPC (docetaxel (11,12), sipuleucel-T (13), cabazitaxel (10), abiraterone (9), 223Ra (14), and MDV3100 (15)), the high observed incidence of marked bone scan improvement with cabozantinib treatment is without precedent. Prominent in vitro targets of cabozantinib are VEGFR2 (in vitro inhibitory concentration of 50%, 0.035 nM) and MET (in vitro inhibitory concentration of 50%, 1.8 nM). There is overlap between the targets of sunitinib and cabozantinib, most notably VEGFR2 (in vitro inhibitory concentration of 50%, 4 nM for sunitinib) but also KIT and RET. The targets responsible for the observed bone scan improvements are not known.
Sunitinib did not improve overall survival for men with metastatic CRPC when it was later studied in a placebo-controlled phase III study (7). Clinical trial experience with targeted therapy for advanced prostate cancer has included targets such as endothelial growth factor receptor (16–19), SRC (20–22), vascular endothelial growth factor (23–26), insulinlike growth factor 1 receptor (27), and human epidermal growth factor receptor 2 (28). To date, no TKI or monoclonal antibody has demonstrated a survival benefit.
The present analysis features several limitations. First, it is a retrospective review of an endpoint (12-wk response by bone scan) that was not specified before the clinical trial and has not been validated in larger studies. Interpretation must therefore be done with caution. Second, the retrospective analysis of an unconventional endpoint in this phase II study is subject to chance observations in a relatively small cohort. Examination of the data from the experimental arm of the completed phase III study of sunitinib in this disease state would be a logical next step. Third, further work is needed to best assess radiographic disease burden and response to treatment in prostate cancer metastatic to bone.
Discordance between bone scan and other disease assessments indicates that osteoblast-based imaging provides an incomplete assessment of treatment-induced changes. Multitargeted TKIs may reduce 99mTc-MDP uptake through tumoristatic or tumoricidal effects, through direct osteoblast inhibition, or through their effects on lesion perfusion. Rational development of TKIs for the management of advanced prostate cancer requires an improved understanding of the mechanistic and clinical significance of treatment-induced bone scan improvements. Key potential future directions include direct tumor imaging with novel PET or SPECT agents and serial imaging of perfusion with either dynamic contrast-enhanced MRI or kinetic modeling of novel tracer uptake.
CONCLUSION
Systematic analysis of images from this phase II trial revealed a relatively high rate of bone scan response without concordant improvements in PSA or CT evidence of disease by accepted criteria. Rational development of multitargeted TKIs for the management of advanced prostate cancer will require an improved understanding of the mechanistic and clinical significance of TKI-induced bone scan improvements.
DISCLOSURE STATEMENT
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by grants from the Dana-Farber/Harvard Cancer Center SPORE in Prostate Cancer, the Prostate Cancer Foundation, and the National Institutes of Health (5K24CA121990-02). The clinical trial (Clinicaltrials.gov identifier: NCT00299741) was funded by a grant from the Department of Defense (DOD) office of Congressionally Directed Medical Research Programs (CDMRP). No other potential conflict of interest relevant to this article was reported.
Footnotes
↵* Contributed equally to this work.
Published online Sep. 14, 2012.
- © 2012 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication February 29, 2012.
- Accepted for publication May 25, 2012.