Abstract
The incidence and prevalence of gastroenteropancreatic neuroendocrine tumors has been increasing over the past 3 decades. Because of high densities of somatostatin receptors (sstr)—mainly sstr-2—on the cell surface of these tumors, 111In-diethylenetriaminepentaacetic acid-octreotide scintigraphy has become an important part of clinical management. 18F-radiolabeled analogs with suitable pharmacokinetics would permit PET with more rapid clinical protocols. Methods: We compared the affinity in vitro and tissue pharmacokinetics by PET of 5 structurally related 19F/18F-fluoroethyltriazole-Tyr3-octreotate (FET-TOCA) analogs: FET-G-polyethylene glycol (PEG)-TOCA, FETE-PEG-TOCA, FET-G-TOCA, FETE-TOCA, and FET-βAG-TOCA to the recently described 18F-aluminum fluoride NOTA-octreotide (18F-AIF-NOTA-OC) and the clinical radiotracer 68Ga-DOTATATE. Results: All 19F-fluoroethyltriazole-Tyr3-octreotate compounds retained high agonist binding affinity to sstr-2 in vitro (half-maximal effective concentration, 4–19 nM vs. somatostatin at 5.6 nM). Dynamic PET showed that incorporation of PEG linkers, exemplified by 18F-FET-G-PEG-TOCA and 18F-FETE-PEG-TOCA, reduced uptake in high sstr-2–expressing AR42J pancreatic cancer xenografts. 18F-FET-βAG-TOCA showed the lowest nonspecific uptake in the liver. Tumor uptake increased in the order 68Ga-DOTATATE < 18F-AIF-NOTA ≤ 18F-FET-βAG-TOCA < 18F-FET-G-TOCA. The uptake of 18F-FET-βAG-TOCA was specific: a radiolabeled scrambled peptide, 18F-FET-βAG-[W-c-(CTFTYC)K], did not show tumor uptake; there was lower uptake of 18F-FET-βAG-TOCA in AR42J xenografts when mice were pretreated with 10 mg of unlabeled octreotide per kilogram; and there was low uptake of 18F-FET-βAG-TOCA in low sstr-2–expressing HCT116 xenografts. Conclusion: We have developed novel fluoroethyltriazole-Tyr3-octreotate radioligands that combine high specific binding with rapid target localization and rapid pharmacokinetics for high-contrast PET. 18F-FET-βAG-TOCA and 18F-FET-G-TOCA are candidates for future clinical evaluation.
- somatostatin receptor
- octreotide
- 18F-fluoroethyl-triazole-Tyr3-octreotate
- positron emission tomography
- and neuroendocrine
The incidence and prevalence of gastroenteropancreatic neuroendocrine tumors (GEP-NETs) has increased significantly over the past 3 decades (1). The most common site of primary GEP-NETs is the gastrointestinal tract (60%). Within the gastrointestinal tract, the single most common site is the small intestine (34%), followed by the rectum (23%), colon (19%), stomach (8%), pancreas (8%), and appendix (7%). There is no standard management of GEP-NETs, although the recent PROMID trial suggested a response benefit in the use of sandostatin LAR Depot (octreotide acetate for injection suspension; Novartis Pharmaceuticals) in patients with metastatic midgut tumors (2). Targeted therapies, including everolimus and sunitinib, are being investigated (3). Treatment of pancreatic neuroendocrine patients with everolimus, for example, has shown prolongation of progression-free survival (4). Other treatment options include radiolabeled somatostatin analogs and surgical debulking; however, no improvements in survival have been reported, and validated biomarkers for response are lacking.
Currently somatostatin-receptor (sstr) imaging is the method of choice for the staging of GEP-NETs. Somatostatin [H-Ala1-Gly2-c(Cys3-Lys4-Asn5-Phe6-Phe7-Trp8-Lys9-Thr10-Phe11-Thr12-Ser13-Cys14)-OH] is a small regulatory peptide that has an inhibitory effect on the production of several exocrine hormones (prolactin, insulin, glucagon, and thyroid-stimulating hormone) in the gastrointestinal tract and antiproliferative effects. sstrs are G-protein–coupled receptors expressed on cell membranes, which on ligand binding generate a transmembrane signal. Five receptor subtypes (sstr-1–sstr-5) have been identified to date (5). GEP-NETs frequently express a high density of sstr, in particular sstr-2, that can be exploited for imaging, from identifying a small primary lesion after biochemical syndrome diagnosis and selecting patients for radiotherapy to exploring the extent of metastatic disease (6–8). Somatostatin analogs have been developed because somatostatin itself has a short plasma half-life (∼3 min). The current somatostatin receptor–based clinical standard for functional imaging of GEP-NETs is scintigraphy with 111In-diethylenetriaminepentaacetic acid (DTPA)-octreotide (half-life of 111In, 2.8 d; photon energy, 254 keV) (9); clinical imaging is then performed at 1–2 d after injection of the radiopharmaceutical to ensure sufficient target-to-background contrast. Octreotide [(d)-Phe1-c(Cys2-Phe3-(d)-Trp4-Lys5-Thr6-Cys7)Thr-ol8] binds with high affinity to sstr-2 and to a lesser extent sstr-5 (10). Substitution of Phe3 by Tyr3, as well as C-terminal Thr(ol)8 by Thr8, gives Tyr3-octreotate [(d)-Phe1-c(Cys2-Tyr3-(d)-Trp4-Lys5-Thr6-Cys7)Thr8], with improved selectivity and affinity for sstr-2, compared with octreotide (8,11,12).
PET offers better image contrast than scintigraphy, and indeed octreotide analogs using radiometals such as 68Ga (1.1-h half-life, 90% β+, 1.9-MeV Eβ+ [positron energy]) and 64Cu (12.7-h half-life, 19% β+, 0.6-MeV Eβ+; also emission of 30% β− particles) have been developed for peptide-based imaging by PET (13–15). A good correlation between the uptake of 68Ga-labeled peptides and sstr-2 immunohistochemistry has been demonstrated in patients, supporting the use of these radioligands for imaging of sstr-2 (16). Peptides labeled with the 18F radioisotope, having the optimal combination of half-life, photon flux, and photon energy (1.8-h half-life, 97% β+, 0.6-MeV Eβ+), should be superior to the metal-based tracers and be devoid of nonspecific uptake resulting from transchelation (17). Consequently, 18F-labeled octreotide analogs have previously been developed (18–20). Tyr3-octreotate has been radiolabeled with both 68Ga and 18F radioisotopes for PET of tumors (21,22). In this article, we explored the relatively new copper-catalyzed azide-alkyne cycloaddition reaction (click reaction) to design several octreotate analogs. Marik and Sutcliffe (23), and Glaser and Årstad (24) were the first to apply click chemistry to 18F radiolabeling of various building blocks or peptides. Since then, the click reaction has been used for rapid chemoselective and regiospecific 18F radiolabeling of peptides under mild reaction condition, with good synthetic yields (25,26). The Tyr3-octreotate core was selected over octreotide because of its superior sstr-2 specificity, higher affinity, and tumor uptake properties (8,12). Rapid tumor accumulation, together with rapid clearance of labeled peptides from normal tissues including the liver and muscle, is a prerequisite for use of short-lived positron-emitting radioisotopes such as 18F. Recognizing that incorporation of polyethylene glycol (PEG) moiety and length or type of linker used to connect the peptide core to the 18F-containing prosthetic group can change peptide disposition, we performed a comparative study to evaluate tissue pharmacokinetics and imaging properties of 5 structurally related 18F-fluoroethyltriazole-Tyr3-octreotate (FET-TOCA) analogs—FET-G-PEG-TOCA, FETE-PEG-TOCA, FET-G-TOCA, FETE-TOCA, and FET-βAG-TOCA (Fig. 1)—and compared these to a scrambled labeled peptide, the recently described 18F-aluminum fluoride NOTA-octreotide (18F-AIF-NOTA-OC (19)) and the clinically used radiotracer 68Ga-DOTATATE. Assessment of the pharmacokinetics and imaging properties in vivo should provide structure–activity relationship data for selection of the optimal radiotracer.
Affinity profiles of different 18F-fluoroethyltriazole-Tyr3-octreotate analogs for sstr subtypes sstr-2 (A) and sstr-4 (B), determined using calcium flux fluorometric imaging plate reader assay as described in “Materials and Methods” section. Assay was performed in duplicate. Fluorescence output was measured and data expressed as percentage maximal fluorescence signal.
MATERIALS AND METHODS
Radiochemicals and Log D Measurements
Five structurally related 19F/18F fluoroethyltriazole-Tyr3-octreotate analogs (Supplemental Fig. 1; supplemental materials are available online only at http://jnm.snmjournals.org) and a related scrambled peptide were prepared. All commercially available chemicals were of analytic grade and used without further purification. Stable 19F was used for assays in which radioactivity was not needed. The synthesis of the radiotracers by click radiochemistry (Supplemental Fig. 1) is reported in detail elsewhere (27). Labeled products were eluted with ethanol in 100-μL fractions and made up to a 10% ethanol/phosphate-buffered saline solution (pH 7.4). 18F-AIF-NOTA was synthesized as previously described (19). 68Ga-DOTATATE was purchased from Covidien (U.K.) Commercial Ltd. The lipophilicity (Log D) of the radiotracers has been reported (27).
In Vitro Binding Assay
Tyr3-octreotate analogs bind mainly to sstr-2. The affinity of stable 19F-fluoroethyltriazole-Tyr3-octreotate analogs for somatostatin receptor subtype sstr-2, versus sstr-4 as control low-affinity-receptor subtype, was determined using a fluorometric imaging plate reader assay. The assay involved measuring the 19F-fluoroethyltriazole-Tyr3-octreotate–induced activation of a calcium flux in sstr-2– or sstr-4–expressing Chem-1 cells (Millipore) that were preloaded with a calcium dye. Briefly, Chem-1 cells expressing a specific sstr subtype were seeded in 96-well plates at 50,000 cells per well and incubated in 5% CO2 for 24 h. Cells were washed and loaded with Fluo-8-No-Wash Ca2+ dye in GPCRProfiler Assay Buffer (Millipore) for 90 min at 30°C in a 5% CO2 incubator. Different concentrations of 19F-fluoroethyltriazole-Tyr3-octreotate or somatostatin (Sigma; positive control) were added, followed by fluorescence determination. The assay was performed in agonist mode in duplicate. Fluorescence output was measured and data expressed as percentage maximal fluorescence signal after baseline correction. The half maximal receptor activation for the various ligands was estimated by sigmoid dose–response fitting using GraphPad Prism (version 4.00; GraphPad Software) for Windows (Microsoft).
Animals and Tumor Models
Six- to 8-wk-old female BALB/c nu/nu athymic mice were obtained from Harlan United Kingdom Ltd. High–sstr-2 pancreatic tumor cell line AR42J (28) and low–sstr-2 human colon cancer cell line HCT116 (LGC Standards) were cultured, respectively, in F12K and RPMI 1640 growth medium containing 10% (v/v) fetal bovine serum, 2 mmol/L l-glutamine, 100 units of penicillin per milliliter, and 100 g of streptomycin per milliliter and grown in a 5% CO2 incubator at 37°C. Tumors were established by subcutaneous injection of 100 μL of phosphate-buffered saline containing 1 × 106 cells. All animal experiments were done by licensed investigators in accordance with the United Kingdom Home Office Guidance on the Operation of the Animal (Scientific Procedures) Act 1986 and within guidelines set out by the United Kingdom National Cancer Research Institute Committee on Welfare of Animals in Cancer Research (29). Tumor dimensions were measured continuously using a caliper, and tumor volumes were calculated by volume = (π/6) × a × b × c, where a, b, and c represent 3 orthogonal axes of the tumor. Mice were used when their tumors reached approximately 200 mm3.
In Vivo Plasma Stability of 18F-FET-βAG-TOCA
Because 18F-FET-βAG-TOCA was the lead compound in the series, we assessed its in vivo stability. 18F-FET-βAG-TOCA (∼3.7 MBq) was injected via the tail vein into non–tumor-bearing BALB/c nu/nu mice. Blood was obtained under general isoflurane anesthesia at 30 min after injection, and plasma samples were prepared and immediately frozen on ice. For analysis, the samples were thawed and kept at 4°C immediately before use. Plasma (∼0.2 mL) was clarified by addition of ice-cold methanol (1.5 mL), followed by centrifugation of the mixture (3 min, 20,000g; 4°C). The supernatant was evaporated to dryness. The residue was resuspended in high-performance liquid chromatography (HPLC) mobile phase (1.2 mL) and clarified (0.2-μm filter), and the sample (1 mL) was injected via a 1-mL sample loop onto the HPLC column. Samples were analyzed by radio-HPLC on a 1100 series HPLC system (Agilent Technologies) equipped with a γ-RAM model 3 γ-detector (IN/US Systems Inc.) and Laura 3 software (Lablogic) and ultraviolet detector (254 nm). A Waters μBondapak C18 reversed-phase column (300 × 7.8 mm) stationary phase was eluted with a mobile phase comprising 67% water (0.1% trifluoroacetic acid)/33% acetonitrile (0.1% trifluoroacetic acid) delivered isocratically at 3 mL/min.
PET Studies
Dynamic PET scans were performed on a dedicated small-animal PET scanner (Inveon PET module; Siemens Molecular Imaging Inc.) as reported previously (29,30). Briefly, tail veins were cannulated under general anesthesia (isoflurane). The animals were placed within a thermostatically controlled environment within the scanner; heart rate was monitored throughout the study. The mice were injected with 3.0–3.7 MBq of the different radiolabeled compounds, and dynamic PET/CT scans were acquired in list-mode format over 60 min. In the case of 18F-FET-βAG-TOCA, blocking studies were also done, whereby radiotracer injection and imaging commenced 10 min after intravenous injection of 10 mg of unlabeled octreotide per kilogram (Sigma) to mice; this dose of octreotide was approximately 100-fold higher than the equivalent dose of unlabeled 18F-FET-βAG-TOCA in the radiotracer injectate. The acquired data in all cases were sorted into 0.5-mm sinogram bins and 19 time frames (0.5 × 0.5 × 0.5 mm voxels; 4 × 15 s, 4 × 60 s, and 11 × 300 s) for image reconstruction. Three-dimensional regions of interest were manually defined on 5 adjacent regions: tumor, liver, kidney, muscle, or urine or bladder (each 0.5 mm in thickness). Data were averaged for tissues at each of the 19 time points to obtain time–activity curves. Radiotracer uptake for each tissue was normalized to injected dose and expressed as percentage injected activity per milliliter of tissue (%ID/mL).
Direct Counting of Tissue Radioactivity
After the 60-min PET scan, a part of the tumor tissue was obtained from mice after exsanguination via cardiac puncture under general isoflurane anesthesia. All samples were weighed and their radioactivity directly determined using a Cobra II Auto-gamma-counter (formerly Packard Instruments) applying a decay correction. The results were expressed as a percentage injected dose per gram (%ID/g).
Statistics
Statistical analyses were performed using Prism, version 4.00 (GraphPad). Between-group comparisons were made using the nonparametric Mann–Whitney test. Two-tailed P values of 0.05 or less were considered significant.
RESULTS
Radiotracers
All radiotracers were successfully prepared with more than 98% radiochemical purity. The total synthesis time was approximately 1.5 h. Log D of the different tracers is shown in Supplemental Figure 1.
In Vitro sstr Subtype Specificity
All the 19F-fluoroethyltriazole-Tyr3-octreotate analogs exhibited agonist activity on sstr-2 (Fig. 1; Table 1), with the scrambled peptide (FET-βAG-[W-c-(CTFTYC)K]) having expectedly poor affinity. The affinity of the ligands (half-maximal effective concentration [EC50]) ranged between 4 and 19 nM (vs. somatostatin at 5.6 nM), with the PEG-TOCA analogs showing the lowest affinity to sstr-2. All 19F-fluoroethyltriazole-Tyr3-octreotate analogs except the scrambled peptide showed detectable activity against sstr-4, but the affinity was poor (≥5.4 mM). None of the compounds exhibited detectable activity against sstr-3 (data not shown).
sstr-2 and sstr-4 Affinities for Various Agonist 19F-Fluoroethyltriazole-Tyr3-Octreotate Ligands Expressed as Concentration at Which Half-Maximal Receptor Activation Occurs (Half-Maximal Effective Concentration, EC50)
18F-FET-βAG-TOCA Stability In Vivo
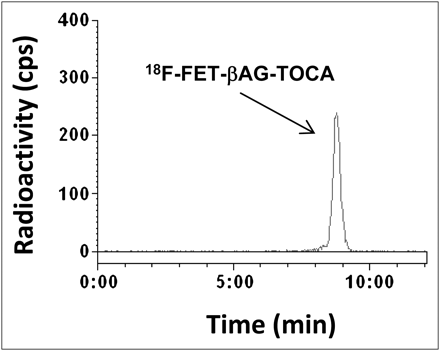
The in vivo stability of 18F-FET-βAG-TOCA was examined. Typical radiochromatograms of dose solution and 30-min mouse plasma are shown in Figure 2. No metabolites of 18F-FET-βAG-TOCA were seen; only intact parent tracer was found.
Radio-HPLC of 18F-FET-βAG-TOCA. Typical plasma extract obtained 30 min after injection of radiotracer into mice, indicating metabolically stable radiotracer. Dose solution was used as reference standard and also showed analyte retention at 8.47 min. cps = counts per second.
Pharmacokinetics and In Vivo Tumor Localization of 18F-Fluoroethyltriazole-Tyr3-Octreotate Analogs
Given the high affinity and systemic stability of 18F-FET-βAG-TOCA, it was reassuring to observe good localization of the radiotracer in the tumor. Figure 3A shows typical transverse and sagittal PET image slices through sstr-2–expressing AR42J tumor–bearing mice, demonstrating localization of 18F-FET-βAG-TOCA in the tumor, kidney, and bladder or urine, with high signal-to-background contrast. In contrast, no radiotracer localization was seen in tumors of the mice when the scrambled peptide 18F-FET-βAG-[W-c-(CTFTYC)K] was injected (Fig. 3B). In this case, tracer localization was seen mainly in the brain, urine, liver, and intestines (data not shown). The comparative pharmacokinetics of all 18F-fluoroethyltriazole-Tyr3-octreotate analogs in the tumor, kidney, liver, and muscle are shown in Figure 4; liver tissue data with expanded y-axis and bladder or urine data are shown in Supplemental Figure 2. Radiotracer uptake in the AR42J tumor was characterized by a rapid increase over the entire scanning period of 60 min. 18F-FET-G-TOCA had the highest tumor uptake, followed by 18F-FET-βAG-TOCA, which had uptake higher than or comparable to 18F-AIF-NOTA-OC. These tracers were superior to the clinical radiotracer 68Ga-DOTATATE with respect to tumor uptake as well as tumor-to-muscle and tumor-to-blood ratios (Table 2). Because the animals were scanned on different days, the mean specific radioactivity for each radiotracer is presented (Table 2). PEG linkers, embodied within the structures of 18F-FET-G-PEG-TOCA and 18F-FETE-PEG-TOCA, reduced tumor uptake but increased tumor-to-muscle and tumor-to-blood ratios (Fig. 4; Table 2). Nonspecific uptake in liver was, in general, low (<7 %ID/mL), with 18F-FET-βAG-TOCA showing the lowest liver uptake; 18F-FET-G-TOCA and 18F-FETE-PEG-TOCA showed the highest liver uptake. The 18F PEG-TOCA analogs had the highest urinary clearance, in keeping with their lower lipophilicity. Radiotracer kinetic profiles in the kidney, which also expresses sstr-2 (31), were different from those in tumors; however, the magnitude of uptake was highest for the 2 radiotracers 18F-FET-βAG-TOCA and 18F-FET-G-TOCA. The mean muscle uptake was less than 3 %ID/mL for all radiotracers. Radiotracer uptake in the bone was low for all the analogs, indicating little or no defluorination. We compared the uptake of the radiotracers in the imaging studies to direct tissue counting. The profiles were generally in agreement, but the magnitude was higher for direct counting, consistent with partial-volume averaging. Direct radioactivity determination (γ-counting) of only a part of the tissue relative to sampling of the whole tumor in the case of imaging could also have led to systematic differences.
PET/CT images showing localization of 18F-FET-βAG-TOCA (A) and scrambled peptide 18F-FET-βAG-[W-c-(CTFTYC)K] (B) in tumors, kidney, and bladder (urine) of AR42J tumor–bearing mice. Transverse and sagittal static (30–60 min, fused; 0.5-mm slice) images are presented.
Time–activity curves comparing tissue pharmacokinetics of 18F-fluoroethyltriazole-Tyr3-octreotate analogs in AR42J tumor (A), kidney (B), liver (C), and muscle (D). Dynamic PET/CT was performed for 60 min after intravenous injection of each radiotracer into tumor-bearing mice. Tissue radiotracer uptake values are expressed as %ID/mL of tissue. Values represent mean ± SEM (n = 3–5); for clarity, only upper or lower error bars are plotted. ° = 18F-FET-G-PEG-TOCA; ● = 18F-FETE-PEG-TOCA; •= 18F-FET-βAG-TOCA; ▪ =18F-FET-G-TOCA; ▴= 18F-FETE-TOCA.
Comparison of Tissue Uptake of 18F-Octreotate Analogs in AR42J and HCT116 Tumor Xenografts
Specificity of 18F-FET-βAG-TOCA Uptake
Given the high tumor uptake of the radiotracers, we next assessed the specificity of uptake in vivo using 18F-FET-βAG-TOCA as the prototypical 18F-fluoroethyltriazole-Tyr3-octreotate. We demonstrated that radiotracer uptake was specific: in keeping with the poor affinity for sstr-2, the radiolabeled scrambled peptide 18F-FET-βAG-[W-c-(CTFTYC)K] did not show detectable tumor uptake in the AR42J model in vivo (Fig. 5). 18F-FET-βAG-[W-c-(CTFTYC)K] uptake was higher in liver than 18F-FET-βAG-TOCA. To show that the tumor uptake of radiotracer was receptor-mediated, blocking studies were conducted by preinjecting mice with an excess of unlabeled octreotide (100-fold molar equivalent) to saturate sstr-binding sites. This resulted in a 2-fold (by direct counting) lower uptake of 18F-FET-βAG-TOCA in AR42J xenografts and even higher reductions in tumor-to-muscle and tumor-to-blood ratios (Fig. 5; Table 2). After blocking with unlabeled octreotide, muscle radioactivity increased. Kidney (early time points only) decreased but subsequently increased; urine radioactivity decreased (Supplemental Fig. 3). Further evidence for the specificity of 18F-FET-βAG-TOCA uptake was provided by the low uptake in low–sstr-expressing HCT116 xenografts, compared with the AR42J xenografts (Table 2).
Specificity of 18F-FET-βAG-TOCA localization in AR42J xenograft model. Kinetics of 18F-FET-βAG-TOCA are shown, along with effect of saturating receptor binding sites with excess cold unlabeled octreotide in tumor (A) and muscle (B). Blocking studies were performed by injecting octreotide (10 mg/kg; intravenously) 10 min before intravenous injection of 18F-FET-βAG-TOCA. Dynamic imaging was performed over 60 min. Tissue radiotracer uptake values are expressed as %ID/mL of tissue. The graphs also illustrate pharmacokinetics of scrambled peptide 18F-FET-βAG-[W-c-(CTFTYC)K] in same mouse model. Values represent mean ± SEM (n = 3–5); for clarity, only upper or lower error bars are plotted. •= 18F-FET-βAG-TOCA in octreotide-naïve mice; ▴ = 18F-FET-βAG-TOCA in mice predosed with 10 mg of unlabeled octreotide per kilogram; ▪ = 18F-FET-βAG-[W-c-(CTFTYC)K in octreotide-naïve mice.
DISCUSSION
We report here new 18F-fluoroethyltriazole-Tyr3-octreotate radioligands that combine high specific binding with rapid target localization for high-contrast PET. Six 18F-fluoroethyltriazole-Tyr3-octreotate analogs were synthesized by the click reaction, and their in vitro affinity and in vivo pharmacokinetics were investigated to enable selection of the optimal radiotracer for PET of somatostatin receptor–positive GEP-NETs. The current somatostatin receptor–based clinical standard for functional imaging, 111In-DTPA-octreotide, suffers from low image contrast and uses a long (1–2 d) diagnostic protocol (9). Substitution of Phe3 by Tyr3 on octreotide, as well as C-terminal Thr(ol)8 by Thr, gives Tyr3-octreotate [(d)-Phe1-c(Cys2-Tyr3-(d)-Trp4-Lys5-Thr6-Cys7)Thr8], with improved selectivity and affinity for sstr-2 (8,11,12). We have now further modified Tyr3-octreotate by introducing an 18F-labeled triazole via the click reaction (24). For these 18F-labeled compounds to be useful, however, they need to combine high tumor uptake with rapid low nontarget washout. We used different chain lengths, alkyl groups, or PEG groups to enable evaluation of the impact of physicochemical variables on pharmacokinetics.
In vitro studies revealed that the triazole analogs had high selective affinity to sstr-2, with half-maximal agonist activity in the calcium flux assay for this G-protein–coupled receptor (EC50) ranging from 4 to 19 nM, compared with the scrambled peptide, which had a low affinity. The combined effect of high binding affinity for sstr and rapid washout from nontarget tissue produced high-contrast PET images in vivo, demonstrated for 18F-FET-βAG-TOCA in Figure 3. The increasing tumor time versus activity curves of 18F-FET-βAG-TOCA derived from the in vivo dynamic PET image data (Fig. 4) reflected the high selective binding of 18F-FET-βAG-TOCA. The radiotracer was metabolically stable in mice and was not defluorinated. Overall, we observed that 18F-fluoroethyltriazole-Tyr3-octreotate radiotracer pharmacokinetics was modified by the structural changes. For example, 18F-FET-G-TOCA that differed in 1 amino acid group (β-alanine) from 18F-FET-βAG-TOCA showed higher tumor uptake and higher nonspecific tissue uptake. This observation may be because of the higher lipophilicity of FET-G-TOCA. Use of ethylene (18F-FET-G-TOCA) instead of amide linker (18F-FETE-TOCA) led to differences in tumor uptake, despite similar sstr-2 agonist affinities in vitro. Thus, chain length and linker type affects pharmacokinetics, but we did not have enough analog data to predict the mechanisms underpinning this effect.
Interestingly, the tumor uptake for 18F-FET-G-TOCA and 18F-FET-βAG-TOCA compounds ranked among the highest reported to date and was higher than that of 68Ga-DOTATATE (Table 1), which is used clinically. Similarly the tumor uptake of the two 18F-fluoroethyltriazole-Tyr3-octreotate analogs was significantly higher than those reported for 111In-DTPA-octreotide by Froidevaux et al. in the same tumor model (3.03 ± 0.26 %ID/g) (32). The tracers also showed uptake similar to or higher than 18F-AIF-NOTA-OC. In contrast to the high tumor uptake of 18F-FET-G-TOCA and 18F-FET-βAG-TOCA, the 2 PEGylated analogs showed lower tumor uptake. Because of reduced glomerular filtration, PEGylation of peptides reduces their overall clearance from the body and increases half-life. We used only small PEG groups of approximately 0.3 kDa, which may not be large enough to affect glomerular filtration. PEGylation also makes compounds more water-soluble (33), supporting faster clearance from the circulation than with the less hydrophilic non-PEGylated analogs. The high urinary clearance of our PEGylated analogs (Supplemental Fig. 2) and high tumor-to-muscle and tumor-to-blood ratios supported the latter mechanism. The low overall tumor uptake of the PEG-TOCA analogs could also be explained by their lower in vitro affinity (Fig. 1; Table 1).
It was predicted that liver tissue that generally shows low 111In-DTPA-octreotide uptake (34) will also show low uptake of the 18F-fluoroethyltriazole-Tyr3-octreotate radiotracers. The PEG-TOCA analogs had lower liver uptake. Interestingly, 18F-FET-βAG-TOCA, with intermediate hydrophilicity, compared with the PEGylated analogs (Supplemental Fig. 1), showed similar low uptake in liver, a positive attribute of 18F-FET-βAG-TOCA that was not realized in 18F-FET-G-TOCA. High nonspecific liver uptake could reduce specificity of imaging agents for detecting metastatic liver disease. 18F-FET-G-TOCA had high liver uptake, making it less attractive for imaging metastatic disease despite showing the highest tumor uptake. Because of its high specificity, characterized by high tumor uptake and tumor-to-muscle ratio but comparatively low background (liver) uptake, we selected 18F-FET-βAG-TOCA for further evaluation.
Evidence from the blocking studies and poor uptake in low-sstr tumors with respect to 18F-FET-βAG-TOCA, as well as low tumor uptake of the scrambled peptide, indicated that the uptake of 18F-FET-βAG-TOCA was receptor-mediated. The in vivo blocking study showed that injecting unlabeled octreotide partially reduced tumor uptake of 18F-FET-βAG-TOCA in the AR42J model. Muscle and liver retained higher radioactivity levels with blocking, possibly because of reduced elimination into urine. Interestingly, the kidney uptake normalized at late time points after blocking. The kidney is a complex organ regarding uptake of somatostatin analogs. The kidney profile observed in our dynamic study may be due to a mix of receptor-mediated and non–receptor-mediated effects (35). For radiometal-labeled octreotide analogs, non–receptor-mediated mechanisms appear to dominate uptake (36). The mechanism for the non–receptor-mediated renal uptake is generally assumed to involve charge-dependent endocytosis in kidneys; in keeping with this assumption, coinfusion of basic amino acids selectively reduces accumulation in kidneys (20,37,38). Other radiometal-labeled peptides including bombesin also show high kidney uptake; however, kidney uptake of labeled peptides is modified by the radioactive label used. Indeed, work by Liu et al. showed that for radiolabeled bombasin, renal uptake decreases in the order 64Cu > 68Ga >> 18F. Therefore, for 18F-octreotate analogs, specific uptake by renal sstr-2 may contribute significantly to uptake, explaining the initial reduction of uptake in kidneys after injection of excess unlabeled octreotide. We also interpret the low renal uptake of the scrambled peptide that had poor in vitro affinity for sstr-2 as resulting at least in part from low receptor binding. Excess cold octreotide may affect renal solute transport. High-dose somatostatin decreases renal plasma flow and glomerular filtration rate in humans (39); the same effect would be expected of other agonists in the mouse given expression of sstr-2 in mouse kidney (31) and could explain the lower urine levels after treatment with cold octreotide. Similarly, we speculate that the smaller than expected reduction in kidney radiotracer uptake at late time points after administration of excess cold ligand could be due in part to reduced renal plasma flow and glomerular filtration. Finally, uptake in AR42J was higher than that in HCT116, in keeping with the higher receptor expression in the former (sstr maximal receptor binding [Bmax], 2,482 fmol of protein per milligram for AR42J vs. 678 fmol of protein per milligram for HCT116 (28,40)). These studies demonstrate specificity of the radiotracers for imaging sstr-2–expressing tumors.
CONCLUSION
We report for the first time, to our knowledge, 18F-fluoroethyl-triazole-Tyr3-octreotate radioligands developed via click chemistry that have good stability, high binding affinity, and optimal pharmacokinetics for imaging sstr-2–positive tumors at early time points. The prerequisite for use of short-lived positron-emitting radioisotopes involving rapid tumor accumulation together with rapid clearance of labeled peptides from normal non–sstr-expressing tissues was met but not through use of the PEG moiety. This unique multiradiotracer design and in vivo assessment has enabled us to select 2 radiotracers, 18F-FET-βAG-TOCA and 18F-FET-G-TOCA, as candidate sstr-2 imaging agents for clinical examination.
DISCLOSURE STATEMENT
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr. Graham Smith for supporting metabolism studies of 18F-FET-βAG-TOCA. We acknowledge funding support from a U.K. Medical Research Council Developmental Pathway Funding Scheme project grant (G0801762) and Cancer Research U.K.–Engineering and Physical Sciences Research Council Centre grant (C2536/A10337). We thank the staff at the Biological Imaging Centre at Imperial College for their support. The authors have filed a patent on this invention. No other potential conflict of interest relevant to this article was reported.
Footnotes
Published online Aug. 18, 2011.
- © 2011 by Society of Nuclear Medicine
REFERENCES
- Received for publication February 3, 2011.
- Accepted for publication May 10, 2011.