Abstract
Dysfunction of the sympathetic nervous system underlies many cardiac diseases and can be assessed by molecular imaging using PET in humans. Small-animal PET should enable noninvasive quantitation of the sympathetic nervous system in mouse models of human disease. For mice, however, the radioactivity needed to give acceptable image quality may be associated with a mass of unlabeled compound sufficient to block the binding of radioligand to its target. The present study assesses the feasibility of using [N-methyl-11C]meta-hydroxyephedrine (11C-mHED) to measure norepinephrine reuptake in humans, to determine cardiac innervation in mice. Methods: Anesthetized mice were placed in a small-animal PET scanner. 11C-mHED (containing 18% precursor metaraminol) was injected via a tail vein into each animal simultaneously. Fifteen minutes later, animals were injected with saline or metaraminol which competes with mHED for norepinephrine reuptake. 18F-FDG was injected at 60 min to identify heart regions. After reconstruction of the list-mode data, radioactivity in myocardial regions was computed using in-house software, and time–activity curves were plotted. Results: Hearts were clearly visualized after injection of 11C-mHED. Injection of metaraminol at doses less than 50 nmol·kg−1 had no effect, whereas doses greater than 100 nmol·kg−1 caused a dose-dependent loss of specifically bound radioactivity. Conclusion: 11C-mHED was successfully used to visualize and assess myocardial innervation in mice. Uptake of 11C-mHED is displaceable by the false transmitter metaraminol. The total molar dose of metaraminol and 11C-mHED must be considered in the analysis of PET data.
- 11C-meta-hydroxyephedrine
- norepinephrine reuptake
- sympathetic innervation
- cardiac imaging
- small-animal PET
The activity of the sympathetic nervous system (SNS) is increased in heart failure because of an increased sympathetic drive and decreased activity and density of the neuronal norepinephrine reuptake transporter (uptake1). As a consequence, β-adrenoceptors are chronically activated, leading to a downregulation in cardiac β-adrenoceptor density (1). Several radioligands are available to investigate the cardiac SNS in patients using PET (2). The most widely used clinically are the norepinephrine mimetic 11C-meta-hydroxyephedrine (11C-mHED) for uptake1 and the nonselective β-adrenoceptor radioligand 11C-CGP 12177 for β-adrenoceptor density. Our group has observed global decreases in both uptake1 and β-adrenoceptor density in nonischemic arrhythmogenic cardiomyopathies (3–5), whereas enhanced reuptake occurred without a change in β-adrenoceptor density in Brugada syndrome (6).
The development of small-animal PET scanners with both a high resolution and a high sensitivity offers the possibility of studying the progression of disease processes in rat and mouse models of human heart failure. In contrast to the wide use of 18F-FDG to assess myocardial metabolism (7), however, there are few published PET studies of myocardial SNS in rodents, although small-animal PET has been used in rats to assess myocardial sympathetic neuronal activity (8) and to evaluate radioligands for β1-adrenoceptors (9).
The use of PET to image molecular targets such as transporters or receptors in small animals imposes challenges not apparent in studies using metabolic tracers such as 18F-FDG, which can be given at the high concentrations needed to achieve good images. Scanner design aims to optimize both resolution and sensitivity, but in dedicated animal scanners resolution is often pursued at the expense of sensitivity so that high doses of radioactivity or long acquisition times are required. With current radiopharmaceutical production methods, high doses of radioactivity are associated with a significant amount of unlabeled compound, which may compromise the specific binding of the radioligand (10).
The quadHIDAC small-animal PET scanner (Oxford Positron Systems) (11) has a good spatial resolution and high sensitivity (12,13). Good-quality images of the mouse heart are achievable using less than 5 MBq of 18F-FDG—which, at a specific activity of 30 GBq·μmol−1, is equivalent to 0.17 nmol, giving 5.6 nmol·kg−1 for a 30-g mouse. At this dose, the specific binding of a high-affinity radioligand, such as (S)-11C-CGP 12177 (in vivo KD ∼1 nmol·kg−1 (14)), is not detectable, whereas specific uptake of radiotracers with moderate affinity, for example, 11C-mHED (in vivo KD ∼100 nmol·kg−1 (15)), is evident.
The aim of the present work was to test the feasibility of assessing myocardial uptake1 in mice using 11C-mHED with the high-resolution quadHIDAC small-animal PET scanner and to develop a quantitative measure of uptake1. With the current radiosynthesis of 11C-mHED, a small amount of the precursor metaraminol is also present in the dispensed 11C-mHED. Metaraminol is a norepinephrine mimetic with an in vivo Ki (∼120 nmol·kg−1) in rat myocardium that is similar to that for mHED (15). Therefore, the effect of metaraminol on myocardial 11C-mHED radioactivity was investigated.
MATERIALS AND METHODS
Radiochemistry
11C-mHED was synthesized by direct N-methylation of metaraminol with no-carrier-added 11C-iodomethane (16). Specific radioactivities at the end of synthesis were 10–30 GBq·μmol−1. Radiochemical purities were 95% ± 5%. For 12 preparations, the mean concentrations of mHED and metaraminol were 9 and 2 μM, respectively.
Pharmaceuticals
Metaraminol bitartrate was purchased from Sigma Aldrich Chemie GmbH. It was dissolved in saline at concentrations of 50 nmol·mL−1 to 10 μmol·mL−1 for injection.
Animals
Studies were approved by the federal animal rights committee and were performed in accordance with institutional guidelines for health and care of experimental animals.
Male C57Bl6 mice (25–35 g) were anesthetized by inhalation (isoflurane; 2%; oxygen, 0.5 L·min−1) for insertion of catheters into a lateral tail vein and, in some animals, the ventral tail artery. Animals were allowed to recover for 1−2 h under light restraint before being reanesthetized for PET. Ex vivo biodistribution studies were performed in conscious animals.
PET
PET was performed using a submillimeter-resolution (0.7 mm in full width at half maximum) dedicated small-animal scanner (32-module quadHIDAC), which uses wire-chamber detectors and offers uniform spatial resolution over a large cylindric field (diameter, 165 mm; axial length, 280 mm) (11–13).
Two or 4 anesthetized mice (isoflurane, 2%; oxygen, 0.5 L·min−1 per mouse) with tail vein catheters were positioned in the scanner lying on their abdomens on a heating pad to maintain body temperature during the scan. Injections were performed via the tail vein catheters using injection loops made from fine-bore polythene tubing and flushed with saline by an infusion pump.
To assess myocardial uptake, 11C-mHED (3−7 MBq in 100 μL per mouse; mHED, 2−90 nmol·kg−1; metaraminol, 1–24 nmol·kg−1) was injected simultaneously into each mouse at 30 s after the start of data acquisition (total injection volume, including saline flush, 200 μL). To assess displacement of 11C-mHED, one mouse was given 50 μL of saline, and the other mouse (or the other 3 mice in the 4-mouse groups) was given 50 μL of metaraminol (0.04−40 μmol·kg−1) at 15 min after injection of 11C-mHED (total injection volume, 150 μL). List-mode data were acquired for 60 min. To confirm the location of the heart, 18F-FDG (4–8 MBq in 50 μL per mouse; total injection volume, 150 μL) was injected via the tail vein at 5−10 min after completion of the 11C-mHED scan, and data were acquired for 30 min.
A similar procedure was used to assess competition between 11C-mHED and metaraminol. Unlabeled metaraminol was added to dispensed 11C-mHED at concentrations to give doses of 0.1−1 μmol·kg−1. The prepared 11C-mHED (100 μL) was injected at 30 s after scan start, and list-mode data were acquired for 60 min. 18F-FDG was then injected to confirm the location of the heart.
Data Analysis
List-mode data were reconstructed into images with a voxel size of 0.4 × 0.4 × 0.4 mm3 in time frames of 10 s, 20 s, 1 min, 10 min, or 20 min for 11C-mHED and 15 min for 18F-FDG, using an iterative reconstruction algorithm (17). PET images were analyzed using in-house software programs in MATLAB (The MathWorks Co.) and C programming languages (13).
To assess the total radioactivity in each animal, a cube encompassing the body, excluding the tail and paws, was drawn on the reconstructed 18F-FDG image (time frame, 15–30 min). The parameters defining this cube were saved and used to compute the whole-body radioactivities (counts per second [cps]·mL−1) for each time frame of the 11C-mHED scan.
The parameters required to create images of each heart and compute time–activity curves were also defined using the 18F-FDG scan (time frame, 15–30 min). The reconstructed volume was divided into 2 or 4 subimages, each showing 1 mouse. From the subimages, coronal images (64 × 64 × 64 pixels) encompassing the heart were made, and regions of interest were drawn manually for myocardium (left ventricular wall and septum) and blood (left ventricular chamber). The parameters were saved and used to create heart images (time frames, 5−15 and 40−60 min) and decay-corrected time–activity curves (cps·mL−1 vs. mid-frame time, for each 10-s, 20-s, or 1-min time frame after injection) for the 11C-mHED scan.
Ex Vivo Studies
11C-mHED (mHED, 2 or 47 nmol·kg−1; metaraminol, 1 or 3 nmol·kg−1) mixed with increasing concentrations of added metaraminol (0.1−10 μmol·g−1) was injected as a bolus (100 μL) via the tail vein. Aliquots of each injectate were diluted in ethanol–saline and measured to determine the radioactivity injected into each mouse. Mice were sacrificed by intravenous injection of sodium pentobarbitone (Narcoren; Merial GmbH) at 200 mg·(kg of body weight)−1 at 30 min after injection. Blood was taken by cardiac puncture and tissues rapidly removed, blotted dry using filter paper, and weighed. Radioactivity was measured using an automated γ-counter (Wallac Wizard 3; Perkin Elmer Life Sciences). To correct for differences in animal body weight and injected dose, results were expressed as an uptake index (15), defined as:
To assess clearance of 11C-mHED radioactivity from the blood, sequential blood samples were collected by dripping blood from a tail artery catheter into a multiwell plate (10-s collection times). Samples were weighed and radioactivity measured using the automated γ-counter. Radioactivity was expressed as cpm·g−1.
RESULTS
PET Scans
11C-mHED in combination with high-resolution small-animal PET resulted in images of the SNS in mice that were comparable to those achieved in humans with respect to resolution and contrast. The heart was no longer visible after the injection of metaraminol, but its position was confirmed by 18F-FDG (Fig. 1).
Distribution of radioactivity after simultaneous intravenous injection of 11C-mHED (3.6 MBq, 22.8 nmol·kg−1) into 2 mice. Fifteen minutes after 11C-mHED, mouse 2 received metaraminol (40 μmol·kg−1 intravenously) and mouse 1 saline. Both mice received 18F-FDG (5 MBq) after 11C-mHED scan. Arrows indicate hearts.
Myocardial Uptake of 11C-mHED
Myocardial images for the mice in Figure 1 are shown in Figures 2A−2F. The late 11C-mHED images (Figs. 2B and 2E) indicated that the apex and part of the septum included spillover from the liver. Therefore, to construct time–activity curves for the myocardium, regions of interest were traced manually round the left ventricular wall and septum (omitting the apical region) on the 18F-FDG image and confirmed on the 11C-mHED images. Results for the left ventricular wall are shown in Figure 2G. The data for mouse 1 were fitted by a biexponential function of the form y = ae−bx + ce−dx. The data for mouse 2 were fitted by a biexponential function before injection of metaraminol and by a single exponential function of the form y = ae−bx + y0 after metaraminol administration. Myocardial radioactivity was detected immediately after injection and reached a maximum during the first minute. The early phase (<5 min) of rapid loss from the left ventricular wall (blood pool) was followed by a slow loss of myocardial radioactivity. The injection of unlabeled metaraminol, but not saline, caused a rapid loss of myocardial radioactivity. It also caused a rapid loss of activity from the ventricular space, an observation consistent with a high spillover from the myocardium (data not shown).
Uptake of radioactivity in mouse heart after intravenous injection of 11C-mHED. (A–F) Images of thoraces of the 2 mice in Figure 1 (scan 1): mouse 1 (A−C); mouse 2 (D−F). Both hearts (arrows) were visualized at 5−15 min after 11C-mHED (A and D). Heart was still visible at 25–45 min after saline (B) but not after metaraminol (E) administration. 18F-FDG confirmed heart positions (C and F). (G) Time–activity curves for left ventricular wall (○, mouse 1; ●, mouse 2). (H) Normalized time–activity curves for scan 1, with results for 2 other scans (scans 2 and 3). In each scan, one mouse was injected with saline at 15 min after 11C-mHED (○, scan 1; Δ, scan 2; ▽, scan 3) and the other with metaraminol (● = 40 μmol·kg−1, scan 1; ▴ = 208 nmol·kg−1, scan 2; ▼ = 100 nmol·kg−1, scan 3). Broken line shows single exponential fit to control data (7 mice) and dotted lines 95% inclusion limits. Solid line shows fits to metaraminol displacement for each dose.
There was a discrepancy between blood time–activity curves derived from PET images and those assessed by continuous arterial blood sampling (Supplemental Fig. 1; supplemental materials are available online only at http://jnm.snmjournals.org). In the absence of corrections for scatter and spillover (Supplemental Fig. 2), it is not possible to obtain an input function by drawing a region in the left ventricle.
Displacement of 11C-mHED by Metaraminol
Because there is no noninvasive method for obtaining an input function, myocardial radioactivity (cps·mL−1myocardium) was normalized to the total radioactivity in the mouse (cpsmouse) for each time frame.
Figure 2H shows normalized myocardial uptake for the 2 mice in Figures 2A−2G, with the results for 2 other pairs of mice. For each pair, one mouse was injected with saline (intravenously) at 15 min after 11C-mHED (control mouse) and the other mouse with metaraminol (test mouse). For clarity of illustration, data points for minute time frames are shown. Metaraminol at 40 μmol·kg−1 or 208 nmol·kg−1 caused rapid loss of myocardial 11C-mHED, but 100 nmol·kg−1 had no significant effect.
Injecting metaraminol at high doses (>500 nmol·kg−1) after 11C-mHED caused a rapid loss of myocardial radioactivity, approximately 70% being displaced during the first 5 min. Therefore, to assess the effect of metaraminol, 10- and 20-s frames were reconstructed for the first 10 min after displacement. Normalized time–activity curves were computed, omitting the first 10-s frame, during which metaraminol was being injected, and using 10-s, 20-s, and subsequent minute time frames.
Normalized time–activity curves for control mice were fitted by a single exponential function
Normalized time–activity curves for test mice were fitted by a single exponential function (Eq. 2) before injection of metaraminol (5–15 min after 11C-mHED) and by a single exponential function with a nondisplaceable background B (Eq. 3) after metaraminol administration (15–50 min after 11C-mHED).
To compare individual control mice, the washout of 11C-mHED radioactivity was expressed as the rate constant b in Equation 2 for 5–50 min after injection and area under the curve (AUC) for 5–50 min (AUC5–50 min). Values for slope b ranged from 0.004 to 0.017 (mean, 0.0122; SE, 0.0015; n = 7), and there was no correlation between the nanomolar dose of norepinephrine mimetics (mHED plus metaraminol) injected (<2–77 nmol·kg−1) and slope b. Values for the AUC5–50 min (mean, 480; SE, 19; n = 7) were not related to injected doses below 35 nmol·kg−1, but 2 animals that received doses of 62 and 77 nmol·kg−1 showed low values (250 and 212, respectively).
For comparison with mice that received displacement metaraminol, data for control mice before or after injection of saline (5–15 min or 15–45 min after 11C-mHED) were also fitted by a single exponential function (Eq. 2). Estimates of rate b and AUCs (AUC for 5–15 min, AUC5–50 min, and AUC for 15–50 min) were not significantly different from those for the fits to data for 5–50 min.
The early washouts of myocardial radioactivity (5–15 min after 11C-mHED) for control (0.013 ± 0.002 min−1, n = 9) or test (0.014 ± 0.003 min−1, n = 12) mice were not significantly different (P = 0.72, Student t test; P = 0.65, paired t test).
Relationships between rate b for 15–50 min after 11C-mHED or AUC for 15–45 min (AUC15–45 min) and metaraminol dose are shown in Figure 3. Results for control mice are plotted against metaraminol (dispensed) injected with 11C-mHED (Figs. 3A and 3C), and those for test mice are plotted against metaraminol (displacement) injected at 15 min after 11C-mHED (Figs. 3B and 3D). The sum of mHED (2–69 nmol·kg−1) and dispensed metaraminol (1–24 nmol·kg−1) ranged from 3 to 104 nmol·kg−1. In some cases, therefore, the amount of norepinephrine mimetic (mHED plus metaraminol) injected at time 0 was greater than the displacement dose of metaraminol given at 15 min (40 nmol·kg−1 to 40 μmol·kg−1).
Displacement of 11C-mHED radioactivity from myocardium by intravenous injection of metaraminol. 11C-mHED injected at time 0 (●, 2–60 nmol·kg−1; ○, 70–90 nmol·kg−1) was associated with metaraminol (dispensed) (1–24 nmol·kg−1). No quality control was available for 1 scan of 2 mice (▴). (A and C) Data for control mice as function of dispensed metaraminol. (B and D) Data for test mice as function of metaraminol (displacement) injected 15 min after 11C-mHED. (B) Solid line shows nonlinear least-squares fit to all data points. (D) Solid line shows nonlinear least-squares fit to data points for which 11C-mHED is ≤60 nmol·kg−1. Broken lines show 95% confidence limits.
For control mice, the rate of loss (0.0092 ± 0.0005 min−1) did not depend on the dose of mimetic injected (Fig. 3A), but displacement metaraminol at doses greater than 100 nmol·kg−1 caused rapid loss (Fig. 3B). The displacement data were fitted to an equation of the form
The AUC15–45 min for control mice (Fig. 3C) indicates that metaraminol doses less than 25 nmol·kg−1 injected with mHED doses less than 60 nmol·kg−1 have no effect on myocardial uptake of radioactivity. Values for test mice, plotted against displacement metaraminol (Fig. 4D), howed a sharp decrease above approximately 50 nmol·kg−1. Mice in 1 experiment received exceptionally high doses of 11C-mHED (68–93 nmol·kg−1). The AUCs for these mice were significantly lower than for mice that received similar doses of metaraminol but lower doses of 11C-mHED and were omitted from further analysis. AUCs for mice that received displacement metaraminol were fitted by a 4-parameter logistic equation:
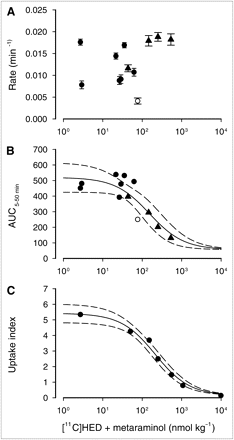
Dose–effect relationships for myocardial uptake of 11C-mHED radioactivity. (A and B) In vivo PET. Saline or increasing doses of metaraminol (100, 200, or 500 nmol·kg−1) were coinjected with fixed amounts of dispensed 11C-mHED (44 nmol·kg−1) and metaraminol (0.8 nmol·kg−1) (▴). Control mice (from Fig. 3) were injected with 11C-mHED (●, 2–60 nmol·kg−1; ○, 70–90 nmol·kg−1) and metaraminol at less than 24 nmol·kg−1. (A) Rates of loss of radioactivity. (B) AUCs. (C) Ex vivo dissection. Saline or increasing doses of metaraminol were coinjected with fixed amounts of dispensed 11C-mHED (2–47 nmol·kg−1) and metaraminol (1–3 nmol·kg−1) into conscious mice. Radioactivity at 30 min after injection expressed as uptake index is shown. Solid lines represent nonlinear least-squares fits to data points and broken lines 95% confidence limits.
Effect of Injected Dose on Myocardial Uptake of 11C-mHED
Studies in rats have shown that myocardial uptake of 11C-mHED is reduced if the combined dose of mHED and metaraminol is greater than approximately 20 nmol·kg−1 and the half-saturation dose is approximately 100 nmol·kg−1 (15). The present displacement studies suggest that these doses are higher in mice (>100 nmol·kg−1). To further investigate this effect, saline or increasing doses of metaraminol were coinjected with a fixed amount of dispensed 11C-mHED and metaraminol. Time–activity curves were constructed using minute time frames, and the rates or AUCs (computed using Eq. 2) were plotted against the combined dose of 11C-mHED and metaraminol (Figs. 4A and 4B). Data for all control mice in Figure 3 are plotted for comparison. There was no correlation between rate of loss of 11C-mHED radioactivity and injected dose. The AUC, however, decreased with doses above 100 nmol·kg−1. Assuming 11C-mHED, mHED, and metaraminol have similar affinities and binding potentials (βmax/Kd) for uptake1 transporters (15), the data were fitted to:
Ex vivo dissection studies gave comparable results (Fig. 4C). 11C-mHED mixed with increasing concentrations of added metaraminol was injected as a bolus via the tail vein into conscious mice. The data were fitted to:
Dose–effect relationships are summarized in Table 1.
Myocardial Retention of 11C-mHED
DISCUSSION
The present study demonstrates that myocardial sympathetic innervation can be visualized in mice using the established tracer 11C-mHED with the high-resolution quadHIDAC small-animal PET scanner. Displaceable specific uptake can be demonstrated and pharmacokinetic parameters measured. This opens an exciting window of opportunity for future work on sympathetic innervation in mouse models of cardiovascular diseases.
The unique advantage of the quadHIDAC scanner is that it offers a high sensitivity and high spatial resolution, which is constant over a large field of view (13). Consequently, several mice may be scanned at the same time, allowing the effects of pharmacologic interventions to be compared using the same radiochemical preparation. This is especially valuable for neuroreceptor systems in which specific uptake of a radioligand is reduced as the amount of unlabeled ligand increases (i.e., as specific activity decreases).
Notwithstanding the limitations imposed by the lack of absolute quantitation in small-animal PET scanners, tracer modeling may give estimates of relevant physiologic parameters. Essentially two methods have been applied to assess myocardial innervation using 11C-mHED in patient studies, both of which require an arterial input function based on serial measurements of blood activity, either by blood sampling or by selecting volumes of interest in a heart chamber (4,18). Serial blood sampling in mice, however, necessitates catheterization of major blood vessels, which is not practical if serial scans are to be obtained to assess the development of disease or therapeutic efficacy. Determining the input function from PET images is an attractive alternative, but spillover from myocardium to the ventricles (blood pool) makes this approach inappropriate for mice. The only option in mice, therefore, was to use a semiquantitative index as previously used in humans (19). Time–activity curves were constructed using normalized myocardial radioactivity (cps·mL−1% cpsmouse). Both washout of 11C-mHED radioactivity and AUCs were calculated.
The PET scans of mice showed changes in myocardial radioactivity (Fig. 2) that were comparable to those after a bolus injection of 11C-mHED in rats, assessed by ex vivo dissection (15,20) or PET (8). An early increase in activity was followed by a phase of rapid decrease (from 20 s to 2 min) reflecting rapid loss from vascular space and then by a phase of slow loss from the myocardium. Metabolite analysis was not performed in this study. In rats, however, although radioactive metabolites were detected in plasma, only parent 11C-mHED was detected in the myocardium (15,20). The data for 5–50 min (Fig. 2) were fitted by a monoexponential function on the basis that loss of 11C-mHED from the nerve terminals is a process that follows first-order kinetics. The rate of loss ranged from 0.04 to 0.176 min−1; there was no relationship between rate of loss and injected nanomolar dose. Values for areas under the time–activity curves (AUC5–50 min) ranged from 400 to 540 for injected doses less than 60 nmol·kg−1.
A bolus injection of unlabeled metaraminol at 15 min after injection of 11C-mHED displaced myocardial radioactivity (Fig. 2), as has been observed ex vivo in rats (15). To assess the effect of metaraminol dose, the time–activity curves after injection of metaraminol were fitted by a monoexponential function with a background. The rate of loss increased from 0.1 to 1 μmol·kg−1 with metaraminol dose and was fitted by Michaelis–Menten kinetics, assuming that displacement by metaraminol is a pseudobimolecular reaction. An alternative measure of the effect of metaraminol was to calculate AUCs after injection of saline or metaraminol (AUC15–45 min, Fig. 3). This parameter showed a sharp decrease as metaraminol dose was increased above approximately 50 nmol·kg−1.
The study in which metaraminol was coinjected with 11C-mHED (Fig. 4) indicated that the rate of loss of myocardial 11C-mHED did not depend on injected dose, whereas the AUC5–50 min showed a clear dependence due to a reduced initial uptake. Comparable results were obtained by ex vivo dissection studies (Fig. 4).
The rate of displacement of 11C-mHED by metaraminol was the parameter least sensitive to metaraminol dose; the dose for half maximum was approximately 300 nmol·kg−1 (Table 1). The competition studies indicated that although the rate of loss of 11C-mHED between 5 and 50 min after injection did not depend on injected dose, the AUC decreased as the total dose of mimetics (11C-mHED + mHED + metaraminol) increased; the half-saturation constant K was 132 ± 59 nmol·kg−1. A similar relationship between uptake index assessed by ex vivo dissection studies and total dose was observed. In this case, the half-saturation constant K was higher (215 ± 32 nmol·kg−1) but not significantly so. The data presented in Figures 3 and 4 indicate that myocardial uptake of 11C-mHED in mice decreases if the total injected dose of mHED and metaraminol is greater than approximately 50 nmol·kg−1. This dose, however, may be lower in mouse models of heart disease because the kinetics of uptake and retention of 11C-mHED depend on the number of neuronal noradrenaline transporters in the myocardium and on their intrinsic activity. A decrease in the number of transporters without a change in their activity will reduce myocardial uptake of 11C-mHED (AUC) but not the half-saturation constant K. A decrease in transporter activity, however, may reduce K. In addition, an increase in endogenous norepinephrine, as is observed in many heart diseases, may reduce the apparent K for injected mimetics.
CONCLUSION
The reuptake of norepinephrine (uptake1) by myocardial sympathetic nerve endings can be successfully visualized in the mouse using 11C-mHED with the quadHIDAC small-animal PET scanner, opening exciting perspectives toward future preclinical studies in mouse models. A semiquantitative index can be used to compare uptake1 in individual mice and gain information about pharmacokinetic parameters. Myocardial uptake of 11C-mHED is dependent on the nanomolar dose of both mHED and metaraminol, the synthesis precursor; a total mimetic dose greater than approximately 50 nmol·kg−1 results in a decrease in myocardial uptake. Consequently, it is essential to ensure that the doses of 11C-mHED plus any metaraminol used with PET to study uptake1 in mice are below approximately 50 nmol·kg−1.
Acknowledgments
We thank Christine Bätza, Irmgard Hoppe, Anne Kanzog, Sandra Schröer, Daniel Burkert, and Sven Fatum for excellent technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft, SFB 656 (projects A5, B2, B3, and Z5), by the Interdisciplinary Center for Clinical Research (IZKF core unit SmAP), Münster, Germany, and by the EU-FP6 project Diagnostic Molecular Imaging (DiMI) (WP 11.1), LSHB-CT-2005-512146.
- © 2010 by Society of Nuclear Medicine
REFERENCES
- Received for publication January 19, 2010.
- Accepted for publication March 24, 2010.