Abstract
The use of PET/CT in children has grown substantially in the past few years. There is also an increased interest in keeping the radiation dose to children from CT as low as is clinically practical. This article reviews the physical aspects of both PET and CT separately and how CT is used in the context of PET/CT to provide the practical insight necessary to approach this issue. Understanding radiation dosimetry and its potential for deleterious health effects, having knowledge of the magnitude of the effective dose and the dose to specific organs from PET and CT, and considering the role of CT in the context of PET/CT will allow the reader to reduce the radiation dose to the patient without compromising the quality of the patient's care.
Since its introduction, PET/CT has revolutionized clinical PET. The incorporation of the anatomic information provided by CT greatly enhances the interpretation of the functional information of PET (1,2). In fact, the major scanner manufacturers no longer offer a PET scanner without a CT component. The addition of CT has been shown to be of particular value in oncology, although it is also useful for cardiology and neurology. The CT data can be acquired as a fully diagnostic scan with the arms kept above the head, imaging during a full breath hold, and use of contrast material for better delineation of tumors and other features. However, if the pediatric patient is anesthetized, there is the potential that maintaining the arms above the head for the duration of the PET/CT scan can cause nerve damage; thus, caution must be applied. In some cases when a recent, diagnostic CT scan is available, the CT component may be used mostly for anatomic correlation of the PET data. Such use of the CT component has been shown in some cases to improve the specificity of PET by eliminating false-positives that result from 18F-FDG uptake in normal tissues such as brown fat.
The CT component is also used for attenuation correction of the PET data. Before the use of CT, measured attenuation correction was determined using transmission scans acquired with rotating radioactive rod sources typically of an equilibrium mixture of 68Ge and 68Ga (3,4). These transmission scans were acquired usually for at least 3 min per bed position; thus, for a whole-body PET scan requiring 6 bed positions, 18 min of the data acquisition was used for acquiring transmission rather than emission data. Conversely, the CT component of the PET/CT scan takes less than 1 min to acquire, thus reducing the acquisition time of the scan by at least 17 min. This reduction in acquisition time can be of particular value in pediatric imaging, possibly reducing the use of sedation or anesthesia. In addition, there is substantially less quantum noise associated with CT-based attenuation correction (CTAC) relative to the use of rotating rod sources.
However, there has been recent discussion about the increased radiation absorbed dose to the population from medical imaging (5). In 1980, medical imaging was estimated to contribute 15% of the average annual radiation exposure received by the U.S. population (0.54 of 3.6 mSv annually) (6). By 2006, this exposure had grown to 50% (3.0 of 6.0 mSv annually) (6). Much of this increase was attributed to the growing use of CT and nuclear cardiology. In 1980, there were 3 and 7 million, respectively, CT and nuclear medicine studies in the United States annually. By 2005, these numbers had grown to 60 and 20 million, respectively (5). The 60 million CT scans accounted for 15% of the radiologic examinations performed, leading to half the cumulative radiation dose to the U.S. population from radiologic procedures (7).
There has been particular concern with respect to the use of CT in children (8–14). Children are known to be more radiosensitive with respect to carcinogenesis than adults because their cells are dividing more rapidly and thereby are more radiosensitive. In addition, children have a potential for a longer life relative to adults and thereby a longer time in which the induced cancer can be realized after irradiation. Both these contributions are discussed in the BEIR VII Report (15). The risk estimate models presented in this report are based in large part on the results of the Life Span Study (LSS) of the survivors from Hiroshima and Nagasaki (16). Forty-one percent of the 86,000 subjects followed in this study were under the age of 20 y at the time of exposure (21% under the age of 10 y). Although the LSS is often considered a high-dose study, 43% of those studied received an estimated dose of less than 5 mSv and 80% under 100 mSv. Hall and Brenner, in their review of the available epidemiologic data including the LSS, noted that the mean dose is about 35 mSv for the lowest dose bin at which an excess relative risk attributed to radiation is statistically significant (12). Table 1 lists the excess attributable risk of death from all solid tumors per 10,000 people per sievert at age 60 y as a function of the age of the individual at the time of exposure based on the models presented in the BEIR VII report (15). According to this table, if 1,000,000 10-y-old children receive 10 mSv, 25 will die as a result of this exposure at the age of 60 y. This risk is 2.1 times higher than that for an individual receiving the same irradiation at an age older than 30 y. In addition, a child receiving the same irradiation at 1 y would have almost 3 times the risk of an individual older than 30 y. For these reasons, the Society of Pediatric Radiology, the American College of Radiology, the American Society of Radiologic Technologists, and the American Association of Physicists in Medicine have worked together for the establishment of the Image Gently campaign to raise awareness on the necessity to maintain pediatric CT doses as low as is appropriate. Other medical associations, including the Society of Nuclear Medicine and the Society of Nuclear Medicine Technologists Section, have also joined the campaign.
Excess Attributable Risk (Deaths) from All Solid Tumors per 10,000 People per Year per Sievert at Age 60 Years
This article will discuss the dosimetry associated with pediatric PET/CT including both the PET and the CT components of the scan. Factors affecting the radiation dose associated with PET, using several radiopharmaceuticals but particularly for 18F-FDG, will be described. Variations in the dosing schemes used in the practice of pediatric nuclear medicine will also be discussed. The dosimetry associated with the CT component will be described in the context of its application with respect to attenuation correction and anatomic correlation. The acquisition of a fully diagnostic CT scan as part of PET/CT will also be considered.
PET DOSIMETRY
The equation for the internal dosimetry from radiopharmaceuticals developed by the Committee for Medical Internal Radiation Dosimetry (MIRD Committee) of the Society of Nuclear Medicine is listed below (16).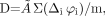 where D is radiation dose to the target organ in grays, Ã is the cumulated activity in megabecquerel-hours, Δi is mean energy per disintegration of the ith radiation in gram-grays/megabecquerel-hours, φi is the absorbed fraction, and m is mass of the target organ in grams. Ã depends on the amount of the radiopharmaceutical administered to the patient, the fraction that went to the source organ, and the time that it resided there. φi is the fraction of the energy emitted by the source organ that is absorbed by the target organ and depends on the type and energy of the radiation and the geometry of the patient. Δi and φi are summed over all radiations emitted by the radiopharmaceutical. Thus, Ã Σ(Δi φi) is the total energy absorbed by the target organ, and dividing this value by the target organ mass yields the radiation dose to the target organ. Therefore, the radiation dosimetry from radiopharmaceuticals depends on the administered activity, patient biology (uptake and clearance rates of activity from the various source organs), patient geometry (organ size, mass, and location relative to other organs), and physics (radionuclide half-life and the number, type, and energy of the emissions).
where D is radiation dose to the target organ in grays, Ã is the cumulated activity in megabecquerel-hours, Δi is mean energy per disintegration of the ith radiation in gram-grays/megabecquerel-hours, φi is the absorbed fraction, and m is mass of the target organ in grams. Ã depends on the amount of the radiopharmaceutical administered to the patient, the fraction that went to the source organ, and the time that it resided there. φi is the fraction of the energy emitted by the source organ that is absorbed by the target organ and depends on the type and energy of the radiation and the geometry of the patient. Δi and φi are summed over all radiations emitted by the radiopharmaceutical. Thus, Ã Σ(Δi φi) is the total energy absorbed by the target organ, and dividing this value by the target organ mass yields the radiation dose to the target organ. Therefore, the radiation dosimetry from radiopharmaceuticals depends on the administered activity, patient biology (uptake and clearance rates of activity from the various source organs), patient geometry (organ size, mass, and location relative to other organs), and physics (radionuclide half-life and the number, type, and energy of the emissions).
In addition to the organ dose, an effective dose is often calculated. To determine the effective dose, the doses to individual organs are calculated, and a weighted sum is calculated for which each organ is weighted by its radiosensitivity. A person receiving a particular effective dose is subjected to the same radiation risk as an individual receiving an equivalent, uniform whole-body dose. In this manner, the concept of the effective dose allows for the comparison of risks for individuals receiving irradiation of a limited number of organs or regions of the body and thus is applicable to those receiving a PET scan for which the radiopharmaceutical may be distributed in only certain organs and a CT scan for which only a limited portion of the body is subjected to the x-ray beam.
The radiation dose to the patient may depend on factors associated with the PET scanner and the scan acquisition and reconstruction because these may affect the amount of administered activity necessary for an adequate diagnostic study. The size of the radiation detector, the proximity of the scanner and detector to the patient, and the scintillation material affect the spatial resolution, sensitivity, and counting rate performance of the scanner and, thereby, the amount of radioactivity necessary to acquire a scan of adequate quality. Another consideration is the maximum amount of time a pediatric patient can remain still to minimize motion artifacts in the reconstructed images. Smaller patients may receive sedation or anesthesia to complete the study, but one might consider a study duration that would reduce the need for such. Whether 2-dimensional or 3-dimensional (3D) PET acquisition is used will also affect the efficiency of the scanner. Two-dimensional PET uses interplane septa that reduce the contributions from interplane scatter and random coincidences from activity outside the field of view, whereas in 3D PET the septa are removed, leading to an improvement in sensitivity of a factor of about 4 or 5 at a cost of increased scatter (scatter fraction increases from 10%−15% to 35%−50%) and random coincidences. Because pediatric patients are generally smaller, the use of 3D PET may be appropriate in many cases. The choice of reconstruction algorithm may also reduce the number of counts necessary for adequate image quality. With iterative reconstruction algorithms such as ordered-subset expectation maximization (OSEM) that model the quantum statistics and the spatial resolution of the scanner appropriately, acceptable image quality for diagnostic interpretation may be achievable with fewer overall counts. Thus, the use of these algorithms may allow for a reduction in either the administered activity or the duration of the acquisition or a combination of both, depending on the clinical task at hand.
18F-FDG is, by far, the most commonly used radiopharmaceutical in PET. Clinical experience has proven its utility for neurology, cardiology, and particularly oncology. Other PET radiopharmaceuticals are used less frequently or are just becoming clinically available. 18F-sodium fluoride (18F-NaF) is used as a bone imaging agent that has been shown to be useful for both oncologic and orthopedic medicine (17). 82Rb (1.3-min half-life) is used at some centers for the imaging of myocardial perfusion. Other PET agents such as 18F-fluorothymidine (18F-FLT), 18F-F-3,4-dihydroxyphenylalanine (18F-DOPA), or 18F-misonidizole are currently being used in some PET clinical research centers and may become commercially available in the future. Table 2 summarizes the radiation dose for several PET agents including 18F-FDG as a function of the age of the patient. The radiation dose to the critical organ (the organ receiving the highest dose in milligrays) and the effective dose are listed for each radiopharmaceutical. The radiation doses from 18F-FDG, 18F-NaF, and 82Rb were estimated using tables from International Commission of Radiation Protection (ICRP) Publication 80 (18). The estimates for 18F-DOPA were calculated using the OLINDA software developed at Vanderbilt University and dosimetric data provided by Brown et al. (19). In Table 2, the administered activity for the different-aged patients is based on patient mass. For a particular radiopharmaceutical using this dosing scheme, the younger patients receive a slightly less effective dose relative to the older patients, but, in general, all of the patients receive roughly the same radiation dose irrespective of age.
Radiation Dose from PET Radiopharmaceuticals
Although there are published guidelines for many nuclear medicine applications as they apply to adults, the practice of pediatric nuclear medicine tends to be more variable from site to site. We surveyed 15 pediatric hospitals in North America with respect to their dosing schemes for radiopharmaceuticals (20). Information was requested on the dosing schemes for 16 commonly performed procedures in pediatric nuclear medicine. Each site reported the administered dose per kilogram, the minimum administered dose, and the maximum administered dose. It was found that the dosing schemes varied substantially from site to site. On average, the administered dose per kilogram and the maximum administered dose varied by a factor of 3 and as much as a factor of 10. On average, the minimum administered dose varied by a factor of 10 and as much as a factor of 20. The concept of minimum administered dose, unique to pediatric nuclear medicine, is the dose below which the study would be deemed inadequate, irrespective of patient size. Some variation in these dosing schemes may be expected because practices may vary with respect to the referral patterns, demographics, and diseases treated. However, factors of 3 or 10 are higher than what might have been expected. The data from this survey regarding 18F-FDG is summarized as follows: the administered dose per unit mass had a median value of 5.37 MBq/kg (range, 5.18–7.40 MBq/kg), the minimum administered dose had a median value of 37 MBq (range, 18.5–74.0 MBq), and the maximum administered dose had a median value of 407 MBq (range, 370–555 MBq) (20).
CT DOSIMETRY
As compared with other radiologic examinations, CT delivers a relatively high radiation dose to the patient, which can be of particular concern when this is combined with the increased radiosensitivity of children for carcinogenesis. Therefore, it is prudent to maintain the radiation exposure from the CT portion of the PET/CT as low as is appropriate while adequately achieving the imaging task at hand. The factors affecting radiation dose in multidetector CT are tube current, rotation times, tube voltage, x-ray beam filtration, pitch (table speed), extent of patient imaged, region of patient imaged, and patient size. The tube current, measured in milliamperes (mA), defines that number of electrons per second that traverse the CT x-ray tube during an exposure and is linearly related to the number of x-rays emitted per second and thereby to the radiation dose rate. Thus, for a particular exposure time, the radiation dose is directly related to the tube current. Conversely, if the duration of the exposure is lengthened by some factor without varying the current, the radiation dose is, in turn, increased by the same factor. Often the tube current and the time are expressed together and given by their product, milliampere-seconds (mAs), because the radiation exposure varies linearly with either parameter. The tube voltage, measured in kilovolt peak (kVp), determines the kinetic energy of the electrons hitting the target. The emitted x-rays have a range of energies (energy spectrum) dependent on the tube voltage and added filtration in the x-ray beam. Increasing the tube voltage increases both the energy range and the height of the continuous x-ray spectrum, and thus the radiation output and thus the entrance dose of the CT scanner varies basically as the square of the tube voltage. Although reducing the tube voltage improves contrast in the image, it also reduces the penetration of each x-ray, requiring an increased number of x-rays entering the patient (i.e., increased mAs) to obtain an adequate number reaching the CT detector. Thus, as the tube voltage is decreased, the milliampere-seconds must be increased to provide the appropriate number of x-rays at the detector to manage the amount of quantum noise in the CT image. These changes increase patient dose. The tube voltage is typically maintained at 120–140 kVp for adult imaging. For small patients, the tube current is sometimes reduced.
Appropriate beam collimation and constraint of the scanned region limits the radiation exposure to the body region of interest while decreasing exposure to tissues and organs outside this region. Exposing these outside regions would increase the radiation risk of the patient, with little or no diagnostic advantage. Although the skin dose within the imaged region may vary only slightly when using a larger scan region, the effective dose (and thereby the radiation risk) increases linearly as the length of the patient irradiated increases because of the exposure of additional tissues and organs. Therefore, it is prudent to limit the CT scan to the specific body region of diagnostic interest.
In helical CT acquisitions, the pitch defines the speed at which the patient's body is advanced through the scanner. The pitch is defined as the length of travel by the imaging table during a single rotation of the x-ray tube divided by the beam collimation. Thus, if the table travels 15 cm during a single rotation and the beam collimation is 10 cm, the pitch would be 1.5:1. The table would travel a distance equal to the beam collimation during an acquisition with a pitch of 1:1. Thus, acquisitions with a pitch less than 1:1 are slightly oversampled in the axial (or z) direction, and those with a pitch greater than 1:1 are slightly undersampled. A higher pitch corresponds to a higher table speed and shorter exposure time for any point of the patient's anatomy. For the same tube voltage and milliampere-seconds, increasing the pitch (and thus the table speed) leads to a corresponding reduction in radiation dose to the patient but also increases the quantum noise in the images.
The size of the patient also affects radiation dose. For a particular set of acquisition parameters (kVp, mAs, beam collimation, and pitch), the skin exposure will be higher for larger patients because the entrance plane is closer to the x-ray source. However, the radiation dose will be reduced at a depth within the patient because of x-ray attenuation. Thus, for a larger patient the radiation dose, compared with skin dose, to the center of the patient will be significantly reduced. Conversely, the radiation beam will undergo less attenuation in a smaller patient, leading to a more uniform radiation dose delivered to the patient. For this reason, the smaller patient receives a higher radiation dose than the larger patient for the same acquisition parameters.
We measured the radiation dose for patients of different sizes as a function of the CT acquisition parameters using a series of anthropomorphic phantoms and an ionization chamber (21). The 5 phantoms varied in size, simulating the torso of a newborn (9 × 10.5 cm, with a circumference of 32 cm); a 1-, 5-, and 10-y-old; and a medium-sized adult (25 × 32.5 cm, with a circumference of 96 cm). These phantoms were made of tissue-equivalent material, with a simulated spine in their posterior portion. There were 5 holes for the potential placement of a pencil ionization chamber. When not in use, these holes can be filled with additional tissue-equivalent material. We measured the radiation exposure from the CT component of a clinical PET/CT scanner (Discovery LS; GE Healthcare). Radiation output from CT scanners is often characterized using the CT dose index (CTDI), which is calculated from exposures measured at various locations within a standard acrylic phantom. Because our anthropomorphic phantoms differed from the standard CTDI phantom, we designated our values as the CT anthropomorphic dose index, to distinguish them from the standard definitions of CTDI.
The resulting data are summarized in Table 3. The linear relationship between radiation dose and milliampere-seconds was assumed in the construction of this table. One can also see the effect that reducing the tube voltage has on radiation dose. For example, considering the phantom data for the 10-y-old acquired at 160 mA, reducing the tube voltage by 33% (from 120 to 80 kVp) led to a 67% reduction in radiation dose (from 14.7 to 4.8 mGy). The table also illustrates that small patients receive a substantially higher radiation dose than do adults for CT scans using the same acquisition parameters. For example, the newborn undergoing CT with 80 kVp and 160 mAs would receive almost twice the radiation dose (20.2, compared with 10.2 mGy) received by an adult undergoing CT with the same parameters. For this reason, the CT acquisition parameters are typically modified according to the size of the pediatric patient. Such a CT dose reduction scheme, proposed by Donnelly et al., in which the amperage is varied as a function of body weight, is given in Table 4 (22).
CT Dose as Function of Patient Age, Tube Voltage, and Tube Current
Acquisition Scheme for Pediatric CT
Table 5 lists estimates of the typical effective dose in adults for various CT scans (13). However, the typical PET/CT scan is acquired from the base of the skull to mid thigh, and thereby, its effective dose may be similar to the combination of the head/neck, chest, abdomen, and pelvis, correcting for the overlap between these scans. In addition to effective doses, it is also important to consider the radiation doses to specific organs. Lee et al. developed computerized phantoms and used Monte Carlo simulation to estimate the organ and effective doses to pediatric patients of different sizes (23). The estimations for a combination of a chest, abdomen, and pelvis (CAP) CT, which is perhaps similar to the CT portion of a PET/CT scan, are summarized in Table 6. In addition, Table 6 also compares the estimation of effective dose for a CAP CT scan with the dose measurements from our phantom experiments, and the estimates are comparable.
Estimated Adult Effective Doses from CT
Estimated Organ and Effective Doses for Pediatric CT
PET/CT DOSIMETRY
The combination of both the PET and the CT portions of the PET/CT scan introduces an added level of complexity, particularly in the context of the CT scan. Specifically, the CT may be primarily used for attenuation correction, or it may be acquired as a fully diagnostic CT scan. In some cases, particularly if a recently acquired CT scan is available, the CT scan may be used only for anatomic correlation of the PET findings rather than directly for diagnosis. Depending on its role, the appropriate level of radiation dose may vary significantly. Therefore, the radiation dose of the PET/CT scan will be reviewed in this context (24).
In CTAC, the reconstructed CT transaxial images are used to estimate and thereby compensate for the amount of photon attenuation in the PET scan. The CT scan is acquired with a tube voltage between 80 and 140 kVp, leading to an effective photon energy in the 40- to 70-keV range, which is then used to correct for the attenuation of the 511-keV photons associated with PET. Although this may seem problematic, a multilinear transformation between the Hounsfield CT values and the linear attenuation coefficient for 511 keV has been shown to be adequate for PET attenuation correction (25). Although the steps may be rearranged, the approach to CTAC is as follows: acquire and reconstruct the registered CT scan; apply the above-mentioned, multilinear energy transformation; reproject these data to generate an attenuation correction matrix; smooth the matrix to the resolution of PET; and apply the correction during reconstruction. The advantages of CTAC over the traditional measured approach using rotating, radioactive rod sources are its speed (acquired in less than 1 min as compared with 15–20 min for the traditional approach) and its significantly reduced noise. On the other hand, CTAC, compared with measured attenuation correction using the rotating rod sources, can deliver a substantially higher radiation dose.
We investigated the adequacy of a low-dose CT scan for CTAC (21). The phantom in Figure 1 was assumed to be the approximate size of the torso of a 10-y-old child. The CT scan on the upper left acquired with 80 kVp, 10 mA, 0.5 and 1.5:1 pitch was used for CTAC in the reconstruction of the PET scan on the lower left, and the CT scan on the upper right (140 kVp, 160 mA, 0.8 s, and 1.5:1 pitch) was used to reconstruct the PET scan on the lower right. The underlying PET data were the same in both cases, and the only difference between the 2 PET images in Figure 1 is which CT scan was used for CTAC during the reconstruction process. The 2 resulting PET reconstructions are essentially identical, even though the 2 CT scans vary significantly with respect to noise. Also, the CT scan on the left would deliver a radiation dose to the patient that is a factor of over 100 less than that of the CT scan on the right. Figure 2 shows the results of a similar experiment performed in a larger phantom that represents a medium-sized adult. In this case, the excessive noise in the CT scan led to an underestimation of the linear attenuation coefficient for the 511-keV photons, which in turn led to an undercorrection. Thus, a tube voltage of 120 keV, while maintaining a low milliampere-second value, was necessary for larger patients for adequate attenuation correction, still leading to a dose reduction of a factor of over 35, compared with that for the CT scan on the right.
Adequacy of CTAC in 20-cm phantom. (A) Transverse CT scan for 80 kVp, 10 mA, 0.5 s/rotation, and 1.5:1 pitch. (B) Transverse CT scan for 140 kVp, 160 mA, 0.8 s/rotation, and 1.5:1 pitch. (C) Transverse PET scan reconstructed using CT scan in A for CTAC. (D) Transverse PET scan reconstructed using CT scan in B for CTAC. Reconstructed PET data in C and D are essentially identical (21). rot = rotation. (Reprinted with permission of (21).)
Adequacy of CTAC in torso phantom. (A) Transverse CT scan for 80 kVp, 10 mA, 0.5 s/rotation, and 1.5:1 pitch. (B) Transverse CT scan for 140 kVp, 160 mA, 0.8 s/rotation, and 1.5:1 pitch. (C) Transverse PET scan reconstructed using CT scan in A for CTAC. (D) Transverse PET scan reconstructed using CT scan in B for CTAC. Use of low-dose CT with larger phantom leads to undercorrection by CTAC (21). rot = rotation. (Reprinted with permission of (21).)
These reductions in radiation dose can be achieved only if the CT scan is used for CTAC and not for anatomic correlation or diagnosis. In addition to pediatric applications, there are a number of instances in which the utility of the CT scan may, in fact, be solely for the purpose of CTAC. For example, in brain PET, for either brain tumors or epilepsy, magnetic resonance is the anatomic imaging modality of choice, and the CT scan is of limited use. Some PET applications such as cardiac imaging with either 13N-ammonia or 82Rb may require multiple acquisitions over an extended period (e.g., stress and rest acquisitions) in which a single CT acquisition at the beginning of the procedure may not be adequate for the CTAC of the later studies. In this case, several low-dose CT scans acquired during the procedure may significantly improve the quality of the quantitation without substantially increasing the radiation dose to the patient. Krishnasetty et al. investigated the use of low-dose CT for CTAC in 35 adult oncologic patients as compared with 43 patients who received their standard weight-based approach (26). The age of the subjects ranged from 26 to 81 y (mean, 61.2 y). The low-dose group received between 0.25 and 0.5 of the radiation dose of the controls, depending on patient size. Thus, their dose reduction in these patients was not as substantial as described above. They reported that although the diagnostic quality of the CT scans was substantially reduced, no difference in image quality was noted in the resultant PET scans.
One may decide to acquire the PET/CT study such that the CT portion can be used as a fully diagnostic scan. In these cases, several logistic and acquisition factors must be considered for the proper interpretation of both scans and the combination of the two. The diagnostic CT scan may require the administration of CT contrast material, possibly adversely affecting the quality of the PET scan by compromising the integrity of the CTAC. An appropriate energy transformation from the Hounsfield CT values to the linear attenuation coefficient for 511 keV and careful use and administration of the contrast material can alleviate such complications in most cases. Respiratory patterns will obviously vary between the CT and the PET portions of the scan because the CT scan can be acquired within a single breath hold (less than 1 min) and the PET scan is acquired over tens of minutes, potentially leading to misregistrations between the 2 datasets—particularly in the inferior lung and the region of the diaphragm. The result can be artifacts within the reconstructed PET scan or misplacements of lesions. Having the patients hold their breath during expiration rather than at end inspiration may help to minimize these effects. Respiratory gating during acquisition of the scan may also be used.
Chawla et al. evaluated the dosimetry of 248 PET/CT scans of 78 pediatric patients with malignancies (27). Fifty-eight percent of the patients in their study did not receive radiation therapy. The effective dose from the CT and PET portions were estimated to have mean values per scan of 20.3 and 4.6 mSv, respectively, and the mean total PET/CT effective dose was 24.8 mSv per scan. There were an average of 3.2 scans per patient (range, 1–14), leading to a mean cumulative effective dose per patient of 78.9 mSv (range, 6.2 and 399 mSv). Twenty-seven percent of the patients received more than 100 mSv. One third of the patients receiving more than 100 mSv did not receive radiation therapy. As discussed previously, these data are higher than listed in Table 5 because of the longer extent (from base of the skull to mid thigh), compared with conventional CT acquisitions. As compared with the effective doses listed in Table 6, these data are most likely higher because of the use of milliampere-second values that are more appropriate for diagnostic-quality CT—that is, higher than 100 mAs.
Even when one chooses to acquire a diagnostic-quality CT scan, it may not be necessary to acquire such for the entire axial extent of the PET/CT scan. The current method is to use a one-size-fits-all approach to PET/CT in which practically all patients receive a high-quality scan from the base of the skull to mid thigh. It may be more appropriate to tailor this approach, depending on the clinical task at hand. For example, if one acquires a PET/CT scan to look for metastases in the lung, it may be most appropriate to acquire a CT scan of diagnostic quality only in the region of the lung and a low-dose CT scan for CTAC for the rest of the scan. If the PET scan reveals uptake in a region outside the lung, a second high-quality CT scan could be acquired after the fact. In most cases, this would lead to a reduction in radiation dose by at least a factor of 2.
In some instances, the anatomic information provided by the CT portion of the PET/CT scan is not used for diagnosis per se but for anatomic correlation of the PET findings. For example, the CT may be used to determine whether an area of increased 18F-FDG uptake is associated with pathology or with an area known for nonpathologic increases in uptake such as brown adipose tissue. If a diagnostic-quality CT scan has already been obtained, it may be possible to substantially reduce the radiation dose and still attain adequate image quality for these sorts of applications. Alessio et al. have investigated the use of a weight-based approach to the acquisition of low-dose pediatric PET/CT scans for anatomic correlation (28). They divided the pediatric population into 11 weight-based categories on the basis of the Broselow–Luten scale originally developed for pediatrics studies performed in the emergency department (29). In this scheme, the CT scan was acquired with 120 kVp and a 1.375:1 pitch; the milliampere-seconds and the 18F-FDG injected activity were varied according to weight category (Table 7). The PET scan was acquired 1 h after injection in 3D mode and reconstructed with 3D OSEM (3 iterations, 28 subsets, and 7-mm postreconstruction smoothing). Alessio et al. estimated the radiation dosimetry associated with the CT by scaling the adult dose to pediatric patients of different ages and weights and used the MIRD model to estimate the PET radiation dose, also listed in Table 7. The authors also estimated the lifetime attributable risk of cancer incidence from these doses to be in the range of 0.1%−0.4%, depending on patient size and sex. In other words, they estimated that these patients may experience an increased lifetime risk of cancer incidence of 0.1%−0.4% as a result of these low-dose PET/CT scans, which can be compared with the baseline lifetime risk of cancer incidence of 37%−46%.
Dosimetry Associated with Weight-Based Approach to PET/CT Scan Acquired for Anatomic Correlation
The radiation doses associated with these scans for anatomic correlation are substantially lower than the estimates for studies with diagnostic-quality CT. For example, Chawla et al. (27) estimated a mean effective dose for diagnostic PET/CT of 24.8 mSv, whereas Alessio et al. (28) estimated an effective dose range of 7.3–11.3 mSv. The authors have noted in a personal communication (October 2008, written communication) that all of the low-dose PET/CT scans acquired with this protocol were of adequate image quality for anatomic correlation. Therefore, if a high-quality CT scan has already been acquired, it may be possible to substantially reduce the patient's radiation dose (by a factor of 2 or 3) by acquiring a low-dose PET/CT scan for anatomic correlation.
CONCLUSION
Epidemiologic studies such as the LSS of the survivors of Hiroshima and Nagasaki have indicated that children are more sensitive to radiocarcinogenesis than adults by perhaps a factor of 2–3, depending on age. In addition, the growing use of CT in children has appropriately heightened the interest in keeping the radiation dose as low as is appropriate. This also follows for the pediatric use of PET/CT, and thus it is prudent for those using the technology to understand the factors that affect radiation dose from both the PET and the CT components of the procedure. One should also consider how the CT portion of the scan is to be used when determining the most appropriate CT acquisition parameters. For example, if one is using the CT only for CTAC, then the radiation dose can be reduced by as much as 2 orders of magnitude, compared with that necessary for a diagnostic-quality CT scan. In addition, if the CT scan is being used for anatomic correlation rather than for direct diagnosis, the radiation dose can possibly be reduced by a factor of 2 or 3. Combining a solid understanding of radiation dosimetry and its associated risk for deleterious health effects, awareness of the magnitude of the effective dose and the dose to specific organs from both the PET and the CT portions of the scan, and knowledge about how the CT scan will be used for specific applications can lead to a substantial reduction in the radiation dose to the patient, without compromising the quality of the patient's care.
Acknowledgments
I thank Matthew Palmer, of the Beth Israel Deaconess Medical Center, and Keith Strauss and S. Ted Treves, of Children's Hospital Boston, for their assistance and support in the preparation of the manuscript.
Footnotes
-
↵* NOTE: FOR CE CREDIT, YOU CAN ACCESS THIS ACTIVITY THROUGH THE SNM WEB SITE (http://www.snm.org/ce_online) THROUGH SEPTEMBER 2010.
-
No potential conflict of interest relevant to this article was reported.
-
COPYRIGHT © 2009 by the Society of Nuclear Medicine, Inc.
References
- Received for publication November 25, 2008.
- Accepted for publication January 7, 2009.









