Abstract
The purpose of this study was to evaluate the role and timing of serial 18F-FDG PET scans as routine surveillance for detecting early locoregional recurrence, distant metastases, and second primary tumors in patients treated for advanced squamous cell carcinoma (SCC) in the oral cavity or oropharynx during the first year after completion of their curative treatment. Methods: Forty-eight consecutive patients with SCC in the oral cavity or oropharynx were included after completing their initial therapy with curative intent. Prospective follow-up of the participants was 2-fold: regular follow-up (history and physical examination) and serial 18F-FDG PET scans. Patients underwent standard follow-up and 18F-FDG PET at 3, 6, 9, and 12 mo after initial treatment. Findings were validated by histopathology or 18 mo of clinical follow-up and imaging after initial treatment. Results: Incidence of recurrences and second primary tumors was 27% and 10%, respectively. 18F-FDG PET was significantly (P = 0.035) more often in agreement with the gold standard than was regular follow-up. 18F-FDG PET showed a sensitivity, specificity, positive predictive value, and negative predictive value of 100%, 43%, 51%, and 100%, respectively. For regular follow-up, these values were 0%, 60%, 0%, and 50%, respectively. 18F-FDG PET accounted for a change in diagnostics or treatment in 63% of the patients and regular follow-up in 25% of the patients. Sensitivity and specificity of 18F-FDG PET were both irrespective of timing of 18F-FDG PET. For the 3- and 6-mo posttherapy results combined, 18F-FDG PET detected malignancy in 16 of the 18 patients. Conclusion: 18F-FDG PET is a suitable routine posttreatment surveillance tool in oral and oropharyngeal SCC patients and detects malignancy before clinical suggestion by the regular follow-up arises. The best timing of a systematic 18F-FDG PET scan is between 3 and 6 mo after treatment.
Despite aggressive combined-modality treatment regimens with curative intent (surgery or radiotherapy or chemotherapy), the locoregional recurrence rate in advanced head and neck squamous cell carcinoma (HNSCC) remains high, up to 45% of the patients (1). Most recurrences occur within the first 2 y after treatment (2). The initial stage of the tumor has been shown to affect the recurrence rate, with stages III and IV having an increased recurrence risk as compared with stages I and II (3). Distant metastases are less frequently occurring, but nevertheless they are reported in approximately 5%−10% of the HNSCC patients. Oropharyngeal squamous cell carcinoma (SCC) more regularly gives rise to distant metastases than SCC from the oral cavity (4). In addition, the risk of a new primary cancer developing in these patients is significantly increased and increases with time (5,6).
In the cases for which tumor recurrence is identified, it is often beyond the stage of salvation. Curative salvage treatment of recurrences and treatment of second primary tumors are possible only if lesions are small and the salvage treatment that is needed is not limited by the earlier performed therapy. Early recognition of recurrent disease and second primary tumors during thorough follow up may allow early salvage treatment and may potentially confer a survival advantage (7).
Effective posttreatment surveillance of HNSCC recurrence is a diagnostic challenge. Postoperative and postradiation changes in the normal tissues may obscure the early detection of recurrence, when conventional follow-up approaches such as clinical assessment, CT, MRI, and endoscopic examination are applied. 18F-FDG PET offers a tool that enables the early detection of HNSCC recurrences. 18F-FDG PET can distinguish recurrent HNSCC from posttreatment changes and is more effective in detecting recurrent tumors than physical examination, CT, or MRI (8–13). However, in most of these studies, the objective was to assess the ability of 18F-FDG PET to visualize a recurrence that was clinically already highly suspected.
18F-FDG PET as a sequential diagnostic tool, independent of whether recurrence is suggested, has been investigated to limited extent. Most of the studies available evaluated the ability of 18F-FDG PET to assess the response of the primary tumor and nodal metastases to radiotherapy or chemotherapy within 2 mo after therapy (14–18). The impact of 18F-FDG PET on subsequent management may be different when searching for cancer recurrence rather than for tumor response. Currently, no consensus exists regarding interval and frequency of PET scans for surveillance of recurrence in HNSCC in subclinical patients after treatment.
The purpose of this study was to evaluate the role and timing of 18F-FDG PET scans as a routine surveillance tool for detecting early tumor recurrence, distant metastases, and second primary tumors in patients treated for advanced SCC in the oral cavity or oropharynx after completion of their curative treatment.
MATERIALS AND METHODS
Patients
This prospective study was conducted at the University Medical Center Groningen and the Medical Center Leeuwarden, The Netherlands. Consecutive patients who had been treated curatively for an advanced (stage III and IV) SCC of the oral cavity or oropharynx were included after completion of their treatment (T0). The patients had to complete a follow-up of at least 18 mo after T0. Not eligible for inclusion were patients treated with palliative intent or without control of disease after treatment.
The study was conducted according to the Dutch Medical Research Involving Human Subjects Act, after approval by the Institutional Review Boards of the participating hospitals. All patients gave written informed consent to participate in the study.
Follow-up Protocol
The study was set up as a nonrandomized paired design. Patients served as their own control (i.e., comparison of PET screening [test] vs. results of regular follow-up [control]). After the patient completed initial therapy, the follow-up of the participant was 2-fold: regular follow-up and serial 18F-FDG PET.
The regular follow-up consisted of history and physical examination at 3-mo intervals at the outpatient department (OPD). The examination included inspection or palpation of all anatomic subsites of the head and neck and an examination of internal structures. Earlier visits to the OPD than scheduled, because of patients' complaints, were considered as a part of the regular follow-up. The outcome of regular follow-up was considered positive on the basis of symptoms or physical signs suggestive of a recurrence or second primary tumor during clinical examination. The clinician had to note if recurrence was suggested. During this assessment, the clinician was unaware of the outcome of the current 18F-FDG PET results.
Besides this regular follow-up, all patients underwent serial 18F-FDG PET investigations at set times (3, 6, 9, and 12 mo) after the completion of initial therapy. 18F-FDG PET was planned on the same day as regular follow-up visits to the OPD.
In the case of negative results of the regular follow-up and serial 18F-FDG PET, no action was planned and a standard follow-up protocol was continued. The study finished 18 mo after T0, leaving a 6-mo observation time after the last PET study.
When local and regional recurrences, distant metastasis, or a second primary tumor were suggested by either regular follow-up or 18F-FDG PET, specific additional diagnostics were performed for confirmation, such as CT, endoscopy, biopsy, cytology, or ultrasound. For any suggestion outside the head and neck area, the patient was referred to the evaluation consultant of the relevant specialty. If a recurrence or a second primary tumor was confirmed by the additional diagnostics, patients were scheduled for palliative or curative therapy.
The outcome of either biopsy or additional diagnostic procedures was the gold standard to compare with positive results of regular follow-up or a 18F-FDG PET scan.
18F-FDG PET Acquisition and Interpretation
All patients had to fast for at least 5 h before undergoing 18F-FDG PET. 18F-FDG was administered intravenously (4–5 MBq/kg). After an uptake period of 90 min, PET emission data were acquired from halfway up the femur to the skull base. Two devices were used: an ECAT EXACT HR + scanner (Siemens CTI), which acquires 63 planes over 15.5 cm, and a Biograph 6 PET/CT scanner (Siemens), which acquires 81 planes over 16.2 cm. The measured resolution of both systems is 4–5 mm in full width at half maximum transaxially in the center of the field of view. On both systems, attenuation-corrected images were obtained, either from low-dose CT data or from a 68Ge/68Ga ring source. CT images were used for attenuation correction only.
Two nuclear medicine physicians, both experienced in PET, visually evaluated all PET images independently. They were unaware of the findings of the current regular follow-up.
In the case of a second, third, or fourth scan, the nuclear medicine physicians had access to all available clinical data at the time of previous scans, including the results of the previous regular follow-up and of morphologic imaging but not of the regular follow-up at the time of the current scan. The level of confidence in image interpretation was graded using a 5-point grading system (0, definitely no tumor; 1, probably no tumor; 2, equivocal; 3, probably tumor; and 4, definite tumor). On a case record form, the results of each scan were divided into 3 regions: primary, neck, and distant. In the final analysis, grades 2, 3, and 4 were considered positive. In the case of discrepancies, consensus was aimed for. If no consensus could be reached, a third independent nuclear medicine physician made the final assessment.
Impact of PET on Patient Management
If a recurrence or second primary tumor was suggested by regular follow-up or 18F-FDG PET, or both, a new diagnostic strategy was applied to the patient. The extent to which 18F-FDG PET and regular follow-up led to changes in management was compared. If the recurrence or second primary could be confirmed within the study period, the change in management was considered to have been appropriate; otherwise, it was considered to have been superfluous.
The number of detected recurrences or second primary tumors by 18F-FDG PET and the regular follow-up were compared and treatment strategies were assessed.
Statistics
A sample size of 40 patients was initially planned. It was estimated that 40% of the patients would have recurrences or second primary tumors. Assuming that 18F-FDG PET had a sensitivity of 80% or 90%, the corresponding 95% confidence intervals would be 57%−93% or 71%−99%, respectively. Dropout was estimated at 20% of all included patients; consequently, 48 patients were required.
Sensitivity, specificity, and negative and positive predictive value of 18F-FDG PET and regular follow-up were calculated on the basis of comparison with the gold standard or with a minimal 6-mo relapse-free time after PET 4 (which refers to PET performed at 12 mo after therapy) with no evidence of malignancy.
Calculations were performed at patient level and scan level for each of the 3 regions separately. The diagnostic value of 18F-FDG PET was compared with that of regular follow-up. At the patient level, comparison was performed by means of the McNemar test. Confidence intervals (95%) of the difference in outcomes were calculated using confidence interval analysis. Proportional observer agreement and Cohen κ were calculated between the nuclear medicine physicians.
RESULTS
Patient Characteristics
Between February 2006 and May 2007, 48 patients were enrolled in the study. All patients (32 men, 16 women) were available for data analysis. Their mean age was 59.9 ± 9.7 y. Tumor characteristics and treatment modalities are listed in Table 1. A reconstruction was performed in 21 patients: split-thickness skin grafts, 8; free radial forearm flaps, 7; free fibula flaps, 5; and pectoralis major flap, 1.
Tumor and Treatment Characteristics of Study Population
Locoregional recurrences, distant metastases, or a second primary tumor after a median follow-up of 7.2 mo (interquartile range, 4.8–13.2) developed in 18 patients (Table 2). During the study, 16 patients died after a median period of 1.6 y (interquartile range, 0.7–1.9 y) after treatment; 15 deaths were due to malignancy, and 1 was due to cardiac arrest.
Characteristics of Recurrences, Distant Metastases, and Second Primary Tumors That Developed in Study Population After Treatment
18F-FDG PET Findings
Patient Level
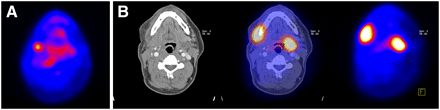
Serial 18F-FDG PET identified all recurrences, distant metastases, or second primary tumors that occurred within the observation period of 18 mo. The regular follow-up detected none at the particular time point yet (Table 2). 18F-FDG PET results were false-positive in 19 patients on 1 or more occasions. Regular follow-up results were false-positive in 12 patients. In 5 of these patients, 18F-FDG PET results were also false-positive. The difference between 18F-FDG PET and regular follow-up is significant (P = 0.035). Table 3 summarizes the diagnostic properties of 18F-FDG PET and regular follow-up. In 10 of 18 patients with a true-positive PET result, diagnostic modalities were capable of confirming the disease directly. In 8 patients, it took at least 3 mo to confirm the diagnosis (Table 2; Fig. 1).
Transaxial 18F-FDG PET/CT images of recurrence with contralateral cervical metastasis. (A) A 3-mo posttreatment 18F-FDG PET image suggestive of local recurrence, not confirmed by physical examination, biopsy, and ultrasound. (B) A 6-mo posttreatment 18F-FDG PET image showing increased 18F-FDG uptake and additional contralateral 18F-FDG focus, confirmed by CT.
18F-FDG PET and Regular Follow-up Performances at Patient Level
Scan Level
In the follow-up period of the study population, 156 scans were performed. All 48 patients underwent a PET scan 3 mo after treatment, 40 patients after 6 mo, 35 patients after 9 mo, and 33 patients after 12 mo.
In Table 4, the diagnostic properties of the serial 18F-FDG PET scans are shown. Overall, 18F-FDG PET showed a high sensitivity and a high negative predictive value. Because of substantial false-positive results, specificity and positive predictive value were considerably lower. Between different anatomic sites (head, neck, and distant), no significant differences were found.
Accuracy of 156 Serial 18F-FDG PET Scans in Detecting Persistent, Recurrent, or Metastatic HNSCC Overall and at Different Anatomic Sites
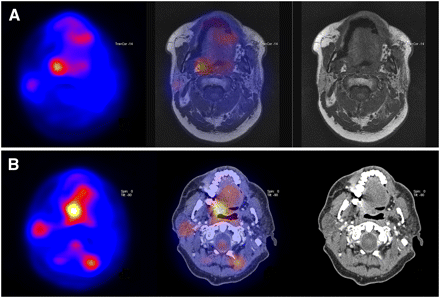
The 35 18F-FDG PET scans that were rated false-positive showed a false-positive hot spot at 38 anatomic sites, 20 of which were local, 6 were regional, 6 were distant, 2 were both local and distant, and 1 was both regional and distant. In addition to these 35 false-positive scans, 1 patient underwent 2 scans, with a true-positive spot in the oropharynx but also a false-positive result for the lung due to an encapsulated fungal infection. Consequently, thirty-seven 18F-FDG PET scans showed 40 false-positive results. In 24 of the 40 (60%) false-positive results, clear (nonmalignant) anatomic substrates, of which the nuclear physicians were not aware at the time of their analysis because clinical data was masked and CT data were not used, were present (Table 5). Correcting for this effect improved specificity and positive predictive value greatly, as shown in Table 4 (data given in parentheses). In 9 patients, the false-positive results had resolved on the next 18F-FDG PET scans. For the remaining false-positive scan results, the false-positive hot spots were present on 2 scans minimally. Three patients showed false-positive hot spots in all 4 scans (Fig. 2), 2 of which were located locoregionally and 1 in the lung.
Transaxial 18F-FDG PET images demonstrated false-positive focus present during all 4 scans in first year after treatment, without confirmation by MRI, CT, and follow-up. Here are shown 3-mo (A) and 12-mo (B) posttreatment images.
Forty False-Positive Results in 37 Scans with Known or Unknown Anatomic Substrate
Proportional observer agreement for detecting malignancy was 0.88, and Cohen κ was 0.75. Per anatomic site, agreement and Cohen κ were 0.87 and 0.65, 0.92 and 0.56, and 0.90 and 0.69, respectively, for the primary site, neck, and distant sites.
Impact of 18F-FDG PET
At the patient level, 18F-FDG PET induced changes in diagnostic procedures or treatment in 63% of the patients, whereas regular follow-up did in 25%. However, the change by 18F-FDG PET or regular follow-up led in 40% and 100% to superfluous diagnostic procedures, respectively—that is, procedures that were performed because of a false-positive result.
In all 18 patients with recurrences or second primary tumors, additional diagnostic procedures for confirmation were initiated by 18F-FDG PET; the regular follow-up initiated none. Seven of the 18 patients (39%) received a curative salvage treatment. Three of these 7 patients died (mean ± SD, 9.5 ± 2.4 mo) after salvage treatment, because of recurrences; 4 are still alive, without signs of malignancy. The other 11 patients received palliative treatment (Table 2).
In 2 patients, 18F-FDG PET led to overtreatment: in 1 patient, lung cancer was suggested by 18F-FDG PET and was confirmed by CT. This patient underwent a lobectomy, but histopathology showed an encapsulated fungal infection. The other patient underwent a neck dissection because of a wrong localization of a local recurrence by 18F-FDG PET.
Timing of 18F-FDG PET Scans
Table 6 shows 18F-FDG PET performances at each time (3, 6, 9, and 12 mo after treatment). Diagnostic properties did not show significant differences among the scans. In 14 of the 18 patients (78%) with disease detected by serial 18F-FDG PET, the disease was recognized on the 3-mo posttreatment scan. Eight were confirmed by additional diagnostic procedures at that time, and 6 patients needed 1 or more diagnostic procedures after PET scans and additional diagnostic procedures to confirm the diagnosis. 18F-FDG PET 3 and 6 mo after therapy detected malignancy in 16 of the 18 patients, including all recurrences. 18F-FDG PET results 9 and 12 mo after treatment were in most patients a confirmation of malignancy detected by previously performed PET scans.
Performance of 18F-FDG PET Scans 3, 6, 9, and 12 Months After Curative Treatment
DISCUSSION
There is a high risk that recurrences, distant metastases, and second primary tumors will develop in patients treated for advanced HNSCC of the oral cavity and oropharynx and will compromise their survival. In the current study, a recurrence rate (local, regional, and distant) of 27% (13 patients) was shown. In 5 patients (10%), a second primary tumor developed. Early identification may allow early treatment with curative intent and may potentially confer a survival advantage (7). We could prove, because of the paired design of the current study, that 18F-FDG PET detects malignancy before clinical suggestion by the regular follow-up occurs during the 1-y follow-up of patients treated for SCC of the oral cavity or oropharynx. Moreover, none of the recurrences and simultaneous second primary tumors was detected by the regular follow-up, and all were detected by 18F-FDG PET.
Currently, there is no consensus regarding the interval and frequency of 18F-FDG PET scans in the follow-up of HNSCC patients. There were several reasons why we chose to perform the first 18F-FDG PET study at 3 mo after treatment. 18F-FDG PET performed within 10 wk after radiation or chemoradiation has been associated with high rates of false-negative findings due to a time period of decreased 18F-FDG uptake after chemoradiation, despite the ongoing presence of viable tumor cells (19). False-positive findings, which are attributed to nonspecific mucosal changes due to chemoradiation, are also associated with 18F-FDG PET performed too early after treatment (20). In addition, the efficacy of chemoradiotherapy (the tumor-kill phenomenon) cannot be fully assessed for at least 8–10 wk after completing chemoradiation. When 18F-FDG PET is performed within 2 mo after treatment, the indication is to evaluate the response to chemoradiation rather than search for recurrences. Moreover, locoregional recurrences, after combined therapeutic modalities including conservative surgery, usually occur later. 18F-FDG PET performed as a sequential investigation 4 mo after treatment, independent of whether recurrence is suggested, showed high accuracy (12,14,21,22). However, recurrences could also be apparent clinically or radiographically at the time of PET. Performing the first sequential PET study at 3 mo after treatment resulted in a high sensitivity and detection rate before other clinical indications were apparent. Similarly, the negative predictive value of 18F-FDG PET was high, in accordance with other studies in which 18F-FDG PET was performed at 12 wk or more after therapy, encouraging an early onset of reconstruction if indicated in case of negative scan findings (23,24).
Few systematic prospective studies have been conducted in which the use of repeated routine posttreatment 18F-FDG PET in a heterogeneous group of HNSCC patients was tested (14,21,25). Because of the study design of repeating scans (every 3 mo), we were able to investigate efficacy and optimum timing of posttreatment 18F-FDG PET scans. Sensitivity and specificity of 18F-FDG PET were not dependent on timing in the 3- to 12-mo posttreatment surveillance (Table 6). 18F-FDG PET at 3 mo detected all recurrences except 1 (Table 2) and induced the greatest change in nonsuperfluous diagnostic strategy. PET 1 and 2 detected malignancy in 16 of the 18 patients and thus, 18F-FDG PET performed at 3–6 mo after therapy, in accordance with the findings of a retrospective study by Lee et al. (26), was the best timing for imaging after treatment. In 2 studies, systematic 18F-FDG PET/CT showed a high accuracy at 12 mo after treatment (27,28). However, the authors suggested a higher impact of 18F-FDG PET at 6 mo after treatment, because the 18F-FDG PET at 12 mo after treatment had significantly less impact than did earlier performed 18F-FDG PET motivated by clinical suspicion (27). Their suggestion was indeed proven by our results. Another study performed routinely 18F-FDG PET/CT about 12 mo after therapy, but with a large SD (positive PET/CT and negative PET/CT results, 10.7 ± 4.7 and 12.3 ± 4.1 mo, respectively) (28).
What is unclear is whether a follow-up 18F-FDG PET scan after a previous systematic 18F-FDG PET scan is indicated and which time interval has to be used. A retrospective study on timing of 18F-FDG PET suggested that locoregional recurrences are unlikely for at least 1 y after initial negative 18F-FDG PET scans (26). Although the impact of a second 18F-FDG PET scan would be significantly less than that of the first systematic 18F-FDG PET scan, it might be appropriate to perform a second PET scan 1 y after the first systematic 18F-FDG PET scan, knowing that in our study 3 second primary tumors and 1 recurrence not detected by the first 18F-FDG PET scan would have been detected at that time point.
A limitation of 18F-FDG PET was its low specificity and positive predictive value. For reasons of screening, a high false-positive risk is more easily accepted as long as sensitivity approaches 100% and false-positive results do not increase the risk of patient morbidity. 18F-FDG PET seems to fulfill these criteria in the current study. Many false-positive scans were related to distinct pathologic lesions other than SCC (Table 5) and in fact were recognized by clinical assessment or other imaging techniques (but were unavailable to the nuclear physician because of the design of the study). However, discrimination between 18F-FDG uptake caused by SCC or by other pathologic lesions is impossible for nuclear medicine physicians unaware of clinical information, and malignancy will be suspected first until proven otherwise. Paradoxically, patients treated for advanced SCC are at high risk for high 18F-FDG uptake both by SCC and by nonneoplastic causes such as mucositis and osteoradionecrosis. In this respect, PET/CT could reduce false-positive results, as it allows direct correlation of 18F-FDG uptake with anatomic structures. PET/CT improves the ability to localize lesions, decreasing the risk of sampling errors (28–30). Moreover, anatomic imaging is required to determine which anatomic structures are involved and to recognize crucial tumor characteristics such as perineural spread, which is related to a poor prognosis and may alter treatment strategies (31). Because of the use of 2 different PET cameras in this study, anatomic information was ignored at first to get a uniform analysis of the study population. Otherwise, a bias could not be excluded because of possible differences in performances between PET/CT and PET fused with separate CT or MRI.
High focal 18F-FDG uptake without a correlating anatomic substrate raises a diagnostic dilemma. In 8 of the 18 patients with true pathologic 18F-FDG uptake, conventional work-up was not able to confirm the 18F-FDG PET findings until 3–12 mo later (Table 2; Fig. 1). In contrast, there were also patients with persistent unexplained 18F-FDG uptake in subsequent scans that was never confirmed (Fig. 2). Therefore, positive 18F-FDG PET scans have to be confirmed by at least 1 other diagnostic procedure to prevent overtreatment. Unfortunately, this compromises the intent to detect recurrences as early as possible. To minimize false-positive results in routine patient care, we recommend the following: nuclear medicine physicians should be informed in detail about clinical history and additional pathology. If there is no explanation for the positive result by clinical assessment, other imaging techniques should be performed. If no anatomic substrate has been found, frequent follow-up and repeated PET after 3 mo are recommended. Figure 3 shows a flow diagram. On the basis of our results, if repeated 18F-FDG PET showed clearly less or no 18F-FDG uptake, a false-positive result for the previous PET study is highly likely. However, if the 18F-FDG uptake was unchanged or increased, discrimination by 18F-FDG PET between true- and false-positive is impossible and morphologic imaging or biopsy is required.
Flow diagram of 18F-FDG PET in HNSCC after treatment. Diagnostic strategy should be revised after twice-repeated 18F-FDG PET to prevent infinite 18F-FDG PET follow-up. neg = negative; pos = positive.
The early detection of recurrent disease or second primary tumors may lead to an improved outcome (32,33). However, despite the early detection, the success rate of salvage treatment in the current study was low; only 7 (15%) patients underwent salvage therapy because of serial 18F-FDG PET, of which 4 (8%) remained free of malignancy. It is not surprising that only a small percentage of the participants could be salvaged, because only a small percentage of patients treated for advanced HNSCC who have a recurrence can be expected to be cured (34). Because the rate of recurrence is highest in advanced HNSCC, these patients were included to study PET effectiveness. To provide data on the impact of systematic 18F-FDG PET on survival, further studies that include patients with lower-staged HNSCC are needed.
CONCLUSION
The current study showed that 18F-FDG PET is significantly more sensitive than regular follow-up for routine surveillance of oral and oropharyngeal SCC patients treated with curative intent. 18F-FDG PET detected all malignancy before clinical suggestions by the regular follow-up existed. In 7 patients (15%), early PET diagnosis led to treatment with curative intent. The impact is highest for 3- and 6-mo posttreatment PET. Therefore, we recommend 1 systematic 18F-FDG PET 3–6 mo after treatment.
Acknowledgments
We give a special thanks to Dr. Adrienne Brouwers and Arjan Vissink for their kind support. We acknowledge the University Medical Center Groningen Stimuleringsgelden for funding this study.
Footnotes
-
COPYRIGHT © 2009 by the Society of Nuclear Medicine, Inc.
References
- Received for publication April 26, 2009.
- Accepted for publication August 17, 2009.