Abstract
Uridine-cytidine kinase (UCK) 2, an enzyme normally expressed in human placenta and testis and highly overexpressed in many neoplasias of blood and solid tissues, catalyzes monophosphorylation of pyrimidine ribonucleosides with efficiency 15- to 20-fold higher than that of ubiquitously expressed isozyme UCK1. In this paper, we report the synthesis of 3′-(E)-(2-iodovinyl)uridine (IV-14) and its preclinical evaluation as a new radiotracer derived from a UCK2-selective antitumor agent, 3′-(ethynyl)uridine. Methods: Radioiodinated IV-14 was prepared from the respective stannyl precursor. 131I-IV-14 was studied in cellular uptake assays and tested for stability in serum as well as for stability to thymidine phosphorylase, liver-, and mucosa-specific murine uridine phosphorylases. UCK1 and UCK2 expression levels in different tumor cell lines were determined by Western blot. Cellular distribution of 131I-IV-14 was determined in HL60 cells. Biodistribution studies and γ-camera scintigraphy were performed on an HL60-xenografted severe combined immunodeficiency (SCID) mouse model. Results: 131I-IV-14 demonstrated excellent stability in serum. It was stable to human thymidine phosphorylase and to liver- and mucosa-specific murine uridine phosphorylases. Cellular uptake after 24 h of incubation with 131I-IV-14 was 4.27 ± 0.21, 3.66 ± 0.13, 2.69 ± 0.07, 2.24 ± 0.18, and 3.26 ± 0.18 percentage injected dose per 5 × 105 Mia-PaCa-2, CX-1, HL60, Capan-1, and Panc-1 cells, respectively. Uptake and retention of IV-14 were regulated by 2 factors: UCK2 expression level and intracellular transport mediated partially via human equilibrating nucleoside transporter 1. A biodistribution study of 131I-IV-14 in an HL60-xenografted SCID mouse model showed that at 4 h after injection the greatest amount of retained radioactivity was in tumor. The tissue-to-tumor ratio 4 h after injection was 1.0 ± 0.24 for tumor, 0.40 ± 0.18 for spleen, 0.25 ± 0.12 for colon, 0.14 ± 0.07 for small intestine, and less than 0.1 for other sites. Scintigraphy with 123I-IV-14 4 h after injection showed the tumor well. In addition, high accumulation of radioiodide in the stomach content was observed and was presumably due to metabolic degradation of IV-14. Conclusion: IV-14 is a UCK2-specific marker, allowing for in vivo addressing of tumors with high RNA synthesis independent of proliferation rate.
Numerous 2′-deoxyribonucleosides have been studied for imaging and radiotherapy of tumors in preclinical and clinical models (1–4). One of them, 3′-deoxy-3′-fluorothymidine (5), is already used in patients as a proliferation marker. However, little is known about the potential of radiolabeled ribonucleosides for application in this field. Only 18F-5-fluorouridine was tested in vitro and in animal tumor models, and it demonstrated unfavorable biodistribution and low metabolic stability (6). The targeting of enzymes related to ribonucleoside metabolic pathways and RNA synthesis could be an attractive alternative to the already investigated targeting of thymidine kinase 1, the key enzyme in the salvage pathway of deoxyribonucleosides and DNA synthesis. DNA synthesis occurs in the S-phase of the cell cycle. In contrast, RNA synthesis occurs in all cell cycle phases except the M-phase. Cellular uptake and metabolism of the marker independently of the cell cycle can be advantageous for imaging and radiotherapy of slowly growing solid tumors, which contain a smaller fraction of the cells in S-phase than do rapidly proliferating tumors. In contrast to 3′-deoxy-3′-fluorothymidine, tracers targeted to RNA synthesis could also potentially be used for visualization of tumors with low thymidine kinase 1 expression.
3′-(Ethynyl)uridine and 3′-(ethynyl)cytidine (Fig. 1) are highly cytotoxic against a broad spectrum of blood and solid tumors, with acceptable toxicity in normal tissues (7). 3′-(Ethynyl)cytidine is currently being tested in phase I clinical trials (8). 3′-(Ethynyl)uridine and 3′-(ethynyl)cytidine in the tumor cell are phosphorylated stepwise to their triphosphates, which inhibit RNA synthesis by nonselective blocking of RNA polymerase (Fig. 1) (9,10). The rate-limiting monophosphorylation step is catalyzed by uridine-cytidine kinases (UCKs) (11), mainly by UCK2 (12). UCK1 is ubiquitously expressed in normal tissues. In contrast, UCK2 is expressed only in the placenta (11) and testis (13). However, UCK2 is expressed in most human cancer cell lines studied (12). In another study (Annette Sommer, written communication, June, 2008), the messenger RNA level of UCK2 was found to be considerably higher in kidney, colon, breast, lung, and ovarian tumor tissues than in the adjacent normal tissues. Notably, uridine and cytosine phosphorylation efficacy is 15–20 times higher by UCK2 than by UCK1 (11). We describe here the preparation and preclinical studies of the radioiodinated analog of 3′-(ethynyl)uridine.
Structures of 3′-(ethynyl)uridine (EUrd) and 3′-(ethynyl)cytidine (ECyt) and metabolic activation of 3′-(ethynyl)uridine and 3′-(ethynyl)cytidine. UMP-CMP kinase = uridine monophosphate/cytidine monophosphate kinase; MP = monophosphate; DP = diphosphate; TP = triphosphate.
MATERIALS AND METHODS
Cell Lines and Reagents
Pancreas Capan-1 carcinoma, colon CX-1 carcinoma, and HL60 leukemia (acute myelogenous leukemia) cells were obtained from DSMZ GmbH. Pancreas BX-PC-3, Mia-PaCa-2, Panc-1, and SK-PC-1 carcinoma cells were generous gifts from Prof. Stephan Hahn (Bochum, Germany). All cell lines were of human origin. Capan-1, CX-1, Mia-PaCa-2, and Panc-1 cells were grown in Dulbecco modified Eagle medium, high-glucose (Life Technologies, GIBCO), containing 10% fetal calf serum (Biochrom), 100 U of penicillin per milliliter, and 100 μg of streptomycin per milliliter (Life Technologies). HL60, BX-PC-3, and SK-PC-1 cell lines were cultured in RPMI 1640 medium (Biochrom) supplemented with 10% fetal calf serum, 100 U of penicillin per milliliter, 100 μg of streptomycin per milliliter, and 2 mmol of glutamine per liter (Biochrom).
Chemicals and solvents were purchased from Sigma-Aldrich, Acrõs, and Merck or from other sources as indicated. All reagents and solvents were of the highest commercially available grade and used without further purification. No-carrier-added sodium 131I-iodide was obtained from Amersham Biosciences, and no-carrier-added sodium 123I-iodide was purchased from Zyklotron AG.
Synthesis of 3′-(2-E)-Tributylstannylvinyl)uridine (TBSVU) and 3′-(E)-(2-Iodovinyl)uridine (IV-14)
Tributyltin hydride (0.298 g, 0.93 mmol) was added to a suspension of 3′-(ethynyl)uridine (0.25 g, 0.93 mmol; prepared from uridine in 5 steps as previously described (14)) in a solution of Pd(PPh3)Cl2 (13 mg, 19 μmol) in dry tetrahydrofuran (30 mL) under argon, and the mixture was vigorously stirred at ambient temperature. More tributyltin hydride was then added (0.22 g [0.75 mmol] and 2 times 0.11 g [0.38 mmol] after 10, 20, and 40 min, respectively), and the 3′-(ethynyl)uridine dissolved gradually to give a clear brown solution. After 1 h, all volatiles were removed under reduced pressure and the crude product was purified by column chromatography on silica gel (first CHCl3 and then 15:3:1 CHCl3:EtOAc:MeOH) to give TBSVU (0.27 g, 52%) as a faint yellow oil. Rf = 0.12 (15:3:1 CHCl3:EtOAc:MeOH) (for the spectral data, see the supplemental materials available online at http://jnm.snmjournals.org).
A solution of iodine (36.3 mg [0.143 mmol]) in CH2Cl2 (3 mL) was added dropwise to TBSVU (80 mg [0.143 mmol]) in CH2Cl2 (3 mL) over a period of 5 min. Thereafter, pentane (12 mL) was added and the precipitate was separated by filtration. The crude product was purified by column chromatography on silica gel (15:3:1 CHCl3:EtOAc:MeOH) to give IV-14 (45 mg, 79%) as a colorless solid. Rf = 0.18 (15:3:1 CHCl3:EtOAc:MeOH) (for the spectral data, see the supplemental materials).
Synthesis of 123/131I-IV-14
Chloramine-T (5 μL, 4 mg/mL in 66% methanolic solution) was added to a reaction vial containing no-carrier-added 123/131I-NaI (4 μL, 159 MBq in 0.05 M NaOH), 0.02 M NaOH (6 μL), 2 M phosphate buffer (10 μL, pH 2, in 30% EtOH), and TBSVU (2 μL of a 2.19 mg/mL solution in 66% MeOH). The reaction was stopped after 5 min by addition of 0.8 M Na2S2O3 (20 μL). 123/131I-IV-14 was purified by semipreparative high-performance liquid chromatography (HPLC) (Rt = 11.8 min; eluent, 10% EtOH; flow, 1 mL/min; column, Kromasil [100 Å, 5 μm, C4, 250 × 4 mm; Eka Chemicals]). For isolation of the product, the fraction between 10 and 13 min was collected. The peak fraction was made isotonic using a 10% NaCl solution and further diluted with isotonic saline to the desired activity concentration. Quality control was as follows: Rt = 3.4 min; eluent, 0.05 M phosphate buffer in 60% MeOH; flow, 4 mL/min; column, Chromolith SpeedRod RP-18e (50 × 4.6 mm; Merck). Radiochemical purity was more than 99%, and the radiochemical yield was 71%−82%.
Serum Stability Assay of 131I-IV-14
To a solution of 131I-IV-14 or 131I-iododeoxyuridine (IdU) (serving as a control) (90 μL, about 7 MBq) were added 10% NaCl (10 μL) and normal human serum (0.9 mL; Sigma), and the mixture was incubated at 37°C. Samples (50 μL) were taken after 0, 10, 30, 60, 120, and 1,440 min and treated with 1.5 M HClO4 (50 μL). The precipitate was separated by centrifugation (10,000 rpm, 4°C, 10 min), and the supernatant was analyzed by HPLC (131I-IV-14: see above; 131I-IdU: Rt = 4.7 min; eluent, 0.05 M phosphate buffer; flow, 4 mL/min; the same column). The radiochemical yields of all reaction products were obtained as the relative peak area fraction of the sum of all areas in the radiochromatogram. Completeness of elution was checked by analyzing the same sample amount choosing a column bypass.
Thymidine Phosphorylase Assay
Susceptibility of IV-14 and IdU to glycosidic bond cleavage by thymidine phosphorylase was studied as follows. To a solution of human recombinant thymidine phosphorylase (10−6−0.1 U; Sigma) in 0.17 mM potassium phosphate buffer (100 μL, pH 7.6), a solution of 131I-IV-14 or 131I-IdU (about 1 MBq) and nonlabeled IV-14 or IdU (3 nmol) in the same buffer (225 μL) was added and the mixture was incubated at 37°C for 30 min. Trifluoroacetic acid (TFA) (30%, 30 μL) was added, and the mixture was cooled to 4°C and analyzed by HPLC as described above.
Uridine Phosphorylase Assay
Susceptibility of IV-14 and uridine to glycosidic bond cleavage by uridine phosphorylases was studied as follows. 131I-IV-14 (2 MBq) was incubated with liver or mucosa homogenate (1.2 mg of protein (15); see the supplemental materials for details on preparation) in 20 mM potassium phosphate buffer (pH 8.0) with 1 mM ethylenediaminetetraacetic acid and 2 mM 2-mercaptoethanol (500 μL) at 37°C for 2 h. The reaction mixture was treated with 1 M phosphoric acid (2 μL), transferred into Microcon YM-3 devices (Millipore) with a cutoff of 3 kDa, and centrifuged at 11,000 rpm and 4°C for 25 min. The supernatant was analyzed by HPLC (eluent A, water [0.1% TFA]; eluent B, acetonitrile [0.1% TFA]; gradient, 0–1 min: 100% A, 10 min: 50% B; 10–15 min: 50% B; flow, 1 mL/min; column, Nucleosil C18 HD [250 × 4.6 mm; Macherey-Nagel]; Rt = 11.3 min for 131I-IV-14, 8.9 min for metabolite 2, and 9.6 min for metabolite 1).
Uridine (8.4 μg, 34.4 nmol) was incubated in the same mixture and under the same conditions. After ultrafiltration, the supernatant was analyzed by HPLC (eluent A, 0.05 M NaH2PO4 [pH 4.15]; eluent B, 50% MeOH in 0.05 M NaH2PO4 [pH 4.15]; gradient, 0–3 min: 100% A, 13.6 min: 100% B; flow, 1 mL/min; column, Gemini C18 [250 × 4.6 mm; Phenomenex]; Rt = 11.3 min for uridine and 7.2 min for uracil; detection, ultraviolet [λ = 254 nm] plus diode matrix array).
Cellular Uptake of 131I-IV-14
Cells (5 × 105/well) were seeded into 12-well plates 24 h before the beginning of the experiment. 131I-IV-14 (100 kBq) in assay medium (1 mL) was then added to the cells and incubated for 2, 4, and 24 h at 37°C and 5% CO2. After incubation, the medium was removed and the cells were washed with ice-cold phosphate-buffered saline (PBS; 2 × 1 mL). Suspension cells were spun down by 1,200 rpm for 5 min at 4°C between the washing steps. The cell pellet was resuspended in 1 M NaOH (1 mL), and the radioactivity was measured in a γ-counter (Cobra II 5003; Perkin-Elmer [Packard]). In the respective experiments, cells (5 × 105/well) were preincubated with 5-fluoro-2′-deoxyuridine (0.01 nM), p-nitrobenzylmercaptopurine (10 μM), or 5-fluorouridine (20 nM) for 1 h before incubation with 131I-IV-14.
Cellular Distribution of 131I-IV-14 in HL60 Cells
A cellular distribution study was performed as previously described for 125I-ITdU in LL/2 cells (16). Briefly, HL60 cells were incubated with 100 kBq of 131I-IV-14 for 2, 4, and 24 h. Thereafter, the cells were washed 2 times with ice-cold PBS and collected by centrifugation. The radioactivity uptake was measured using a γ-counter. Afterward, the cells were homogenized in ice-cold 0.2 M HClO4 (1 mL). After being cooled on ice for 10 min, the homogenates were centrifuged for 10 min at 1,200g and 4°C to give a supernatant containing the acid-soluble fraction and a precipitate. The precipitate was resuspended with Type III Ribonuclease (Sigma) solution (2 mL of a 0.66 mg/mL solution) and incubated at 37°C. After 30 min, ice-cold 5 M HClO4 (0.5 mL) was added. The probes were cooled on ice and centrifuged. The RNA-containing supernatant was separated, and the precipitate was resuspended in ice-cold 1 M HClO4 (1 mL) and incubated for 15 min at 90°C. After cooling and centrifugation, the DNA-containing supernatant was separated and the remaining protein and lipid fractions were washed once with 1 M ice-cold HClO4 (1 mL) and resuspended in ice-cold 1 M NaOH (1 mL). The radioactivity of all samples was measured using a γ-counter. The purities of the isolated DNA and RNA fractions were measured at 2 wavelengths, 260 and 280 nm, and the ratios of the 2 absorbencies were calculated. The A260/A280 ratio was about 2.0 for RNA and between 1.7 and 1.9 for DNA.
Immunoblot Analysis
For analysis of UCK2/UCK1 expression in tumor cell lines and mouse tissue, total protein lysates (spleen, small intestine, liver, and HL60 xenograft) were prepared by lysis with Tris·HCl buffer (50 mM; pH 7.4; NaCl, 300 mM; ethylenediaminetetraacetic acid, 2 mM), NP-40 (1%), phenylmethylsulfonyl fluoride (1 mM), and inhibitor cocktail (Roche). The protein samples were boiled for 5 min in reducing Laemmli buffer supplemented with 5% 2-mercaptoethanol. Equal amounts of protein were subjected to electrophoresis (10% Tris·HCl gel; Bio-Rad) and blotted onto polyvinylidene difluoride membrane (Bio-Rad). Immunostaining of UCK2 was performed with chicken polyclonal anti-UCK2 antibody (1:2,000; Abcam) and immunostaining of UCK1 with rabbit polyclonal anti-UCK1 antibody (1:2,000; Abgent). The secondary goat antichicken IgY (1:2,000, Abcam) and goat antirabbit IgG (1:5,000, Dianova) antibody coupled to horseradish peroxidase antibody were visualized with enhanced chemoluminescence (ECL+; GE Healthcare) as recommended by the supplier. Equal protein loading was controlled using glyceraldehyde 3-phosphate dehydrogenase (GAPDH)–specific antibody (1:5,000; Ambion) and secondary goat antimouse IgG linked to horseradish peroxidase (1:2,000; Abcam).
Flow Cytometric Assay of Human Equilibrating Nucleoside Transporter 1 (hENT1) Expression on Tumor Cells
Cell suspensions were diluted to 1 × 106 cells/mL with PBS for assay of total binding or with 5 μM p-nitrobenzylmercaptopurine in PBS for the assessment of nonspecific binding of the probe and kept at room temperature for 30 min. 5′-[(Fluorescein-5-ylcarbonyl)-6-aminohexanoyl-(2-aminoethyl)]-N6-(4-nitrobenzyl)-5′-thioadenosine (SAENTA-x8-fluorescein; solution in 4% dimethyl sulfoxide) (17) was then added to a 50 nM final concentration, and incubation was continued for a further 20 min at room temperature. The cells were washed once with PBS, and the cell-associated fluorescence was measured using a FACSCalibur Flow Cytometer (BD Biosciences). Fluorescence signals from 10,000 cells were analyzed to determine the mean fluorescence intensity.
In Vitro and In Vivo Metabolite Analysis
After the incubation for 2, 4, and 24 h with 131I-IV-14 (0.5–3.0 MBq), the Mia-PaCa-2 cells (with or without 5-fluorouridine [20 nM] pretreatment for 1 h) were lysed as described above. After centrifugation at 10,000 rpm and 4°C for 10 min, the clear supernatant either was directly purified by ultrafiltration using Microcon YM-30 devices with a cutoff of 30 for 40 min at 12,000 rpm and 4°C to remove protein residues or was first treated with calf intestinal alkaline phosphatase (1 U/μL; Invitrogen) as follows. The supernatant (350 μL) was charged with “10-fold dephosphorylation buffer” (50 μL, 50 mM Tris·HCl [pH 8.5], 0.1 mM ethylenediaminetetraacetic acid) and calf intestinal alkaline phosphatase (100 μL, 100 U) and subsequently incubated for 2 h at 37°C. Thereafter, samples were purified by ultrafiltration as described above. In vivo metabolite analysis was performed on animal urine, blood, stomach and stomach content, small intestine, liver, and tumor obtained from HL60-xenografted severe combined immunodeficiency (SCID) mice. Blood or urine samples (50 μL) were charged with 200 μL of aqueous heparin (100 IE). Liver, tumor, small intestine, and stomach (100 mg) were homogenized in 10 mM sodium-potassium phosphate buffer (pH 6.8) with a Micro-Tissue homogenizer (Wheaton). Thereafter, samples were prepared as described above. Samples were analyzed by radio-HPLC. (Condition A: eluent A, water [0.1% TFA]; eluent B, acetonitrile [0.1% TFA]; gradient, 0–1 min: 100% A, 10 min: 50% B, 10–15 min: 50% B; flow, 1 mL/min; column, Nucleosil C18 HD [250 × 4.6 mm; Macherey-Nagel]; Rt = 11.2 min for 131I-IV-14, 8.7 min for metabolite 1, 5.0 min for metabolite 2, 4.4 min for metabolite 3, and 4.0 min for 131I−. Condition B: eluent A, water [0.1% TFA]; eluent B, acetonitrile [0.1% TFA]; gradient, 0–12 min: 100% A, 20 min: 100% B; flow, 1.8 mL/min; column, Gemini-NX C18 [250 × 4.6 mm; Phenomenex]; Rt = 17.2 min for 131I-IV-14, 15.9 min for metabolite 1, 14.8 min for metabolites 2 and 3, and 2.3 min for 131I−. Condition C: eluent A, water [0.1% TFA]; eluent B, acetonitrile [0.1% TFA]; gradient, 0–1 min: 100% A, 10 min: 50% B; 15 min: 50% B; flow, 1.0 mL/min; column, Gemini-NX C18 [250 × 4.6 mm; Phenomenex]; Rt = 11.7 min for 131I-IV-14, 9.6 min for metabolite 1, 8.9 min for metabolite 2, 8.2 min for metabolite 3, and 4.0 min for 131I−.) Metabolite 1 had a retention time identical to that of IV-14 monophosphate (for details on preparation, see the supplemental materials; Supplemental Fig. 1 compares metabolite 1 and nonlabeled IV-14 monophosphate by HPLC).
Tumor Model
A suspension of 4 × 106 HL60 cells was injected subcutaneously into the scruff of the neck, between the shoulders, of 6-wk-old male and female C.B-17 SCID mice (Taconic). After 3 wk, subcutaneous tumors about 20–30 mm in diameter developed. All animal experiments were performed in accordance with national and local regulations for animal and radiation protection and good experimental practice.
Biodistribution Study of 131I-IV-14 in HL60-Xenografted SCID Mouse
Five megabecquerels of 131I-IV-14 in saline (200 μL) were injected into the lateral tail vein of C.B-17 SCID mice bearing xenotransplanted HL60 tumors. The mice were sacrificed at 1, 4, and 24 h after injection by cervical dislocation. Tumors and organs were excised, weighed, and assayed for radioactivity in a γ-counter. The contents of the stomach, small intestine, and colon were removed before weighing and measurement. Mean tumor and organ uptake was determined from half-life–corrected tissue radioactivity normalized to injected dose and tissue sample weight (percentage injected dose per gram of tissue [wet weight]).
Scintigraphy with 123I-IV-14 in HL60-Xenografted SCID Mouse
Imaging was performed using an e.cam γ-camera (Siemens Medical Solutions) coupled with a low-energy high-resolution collimator. Images of mice were recorded 4 h after IV application of 20 MBq of 123I-IV-14 under xylazine (16 mg/kg) and ketamine (100 mg/kg) anesthesia for 30 min using a 1,024 × 1,024 matrix, a zoom factor of 2 (pixel size, 0.3 mm), and a 15% energy window centered at 159 keV.
Statistical Analysis
Uptake in the different cell lines was compared using the Mann–Whitney U test. Differences were considered to indicate statistical significance for a 2-tailed P value of less than 0.10.
RESULTS
Radiosynthesis of No-Carrier-Added 123/131I-IV-14
123/131I-IV-14 was obtained from TBSVU in 71%−82% radiochemical yield (n = 12) and in greater than 99% radiochemical purity. TBSVU was in turn stereo- and regiospecifically synthesized from 3′-(ethynyl)uridine according to the Guibe–Zhang protocol (18) in 52% yield (Fig. 2A) and was treated with iodine to give nonlabeled IV-14 in 79% yield.
Synthesis of TBSVU, IV-14, and 123/131I-IV-14. EUrd = 3′-(ethynyl)uridine; RP = radiochemical purity; RCY = radiochemical yield.
Stability in Serum and Susceptibility to Thymidine Phosphorylase– and Uridine Phosphorylase–Mediated Cleavage
131I-IV-14 was stable in serum for at least 24 h. In the same period, IdU decomposed to about 30% of the initial quantity (Supplemental Fig. 2). 131I-IV-14 was also resistant to hydrolysis by thymidine phosphorylase, even at an enzyme concentration, which caused complete hydrolysis of IdU (Supplemental Fig. 3). 131I-IV-14 was also resistant to degradation mediated by the murine liver–specific uridine phosphorylase (liver homogenate was used as a source of enzyme). Incubation of 131I-IV-14 with murine mucosa homogenates as a source of murine intestine–specific uridine phosphorylase caused only insignificant hydrolysis (<2%). Under the same conditions, 47% and 90% of the initial quantity of uridine was hydrolyzed by liver– and intestine–specific uridine phosphorylases, respectively, was hydrolyzed into uracil (data not shown).
Cellular Uptake Studies
131I-IV-14 showed significant uptake into all tumor cell lines tested, except BX-PC-3 and SK-PC-1 (Fig. 3A). Both cell lines had low UCK2 expression and a low number of hENT1 transporters on the cell surface. Consequently, the uptake was high for Mia-PaCa-2, Panc-1, Capan-1, and CX-1 cells expressing UCK2 at a high or moderate level (Fig. 3B) (0.79%−2.24% at 2 h, 1.14%−2.30% at 4 h, and 2.24%−4.27% at 24 h). Concomitantly, an appreciable number of hENT1 transporter sites on the surface of these cells were detected by fluorescence-activated cell sorting with 5-(SAENTA-x8)-fluorescein, a cell membrane–impermeable inhibitor of hENT1 nucleoside transporters (Supplemental Fig. 4) (19). After 24 h of incubation, the accumulation of radioactivity in HL60 cells with relatively low UCK2 expression but with the highest number of extracellular hENT1 transporters among cell lines studied was significant (2.69% ± 0.07%) and even greater than in Capan-1 cells with higher UCK2 and lower hENT1 expression (2.24% ± 0.2%).
Cellular uptake of 131I-IV-14 (100 kBq) in different tumor cell lines (A) and Western blot analysis of UCK1 and UCK2 expression in tumor cell lines studied (B). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression is given as loading control.
It was reported (20,21) that pretreatment with nontoxic concentrations of the thymidylate synthase inhibitor such as 5-fluoro-2′-deoxyuridine increased cellular uptake of 2′-deoxynucleosides such as ITdU and IdU in tumor cells. In contrast, uptake of 131I-IV-14 in HL60 and Mia-PaCa-2 cells (Fig. 4A) was not increased by preincubation with 5-fluoro-2′-deoxyuridine. At the same time, uptake was partially inhibited by p-nitrobenzylmercaptopurine riboside, which is a highly selective inhibitor of hENT1 nucleoside transporter (22), in Mia-PaCa-2 and HL60 cells (up to 40% and 83% inhibition after 24 h, respectively). Uptake of 131I-IV-14 in both cell lines after 2 h of incubation diminished significantly after preincubation with 5-fluorouridine (up to 64% and 61% for Mia-PaCa-2 and HL60 cells, respectively). However, this effect was much less pronounced after 4 h of incubation. After 24 h, uptake in 5-fluorouridine–pretreated cells was virtually the same as in control cells. Uptakes under the different inhibition conditions after 2 h of incubation were statistically different (P < 0.1) in all cases. The only exception was uptake in HL60 cells with and without 5-fluoro-2′-deoxyuridine.
(A) Dependence of cellular uptake of 131I-IV-14 in HL60 and Mia-PaCa-2 cells on pretreatment with 5-fluoro-2′-deoxyuridine (FdUrd), 5-fluorouridine (FUrd), and p-nitrobenzylmercaptopurine (NBMPR). (B) Cellular distribution of 131I-IV-14 in HL60 cells. (C) Metabolism of 131I-IV-14 by Mia-PaCa-2 cells (M-1 = metabolite 1; M-2 = metabolite 2; M-3 = metabolite 3). (D) Hydrolysis of M-1–M-3 by calf intestinal alkaline phosphatase (CIAP).
In HL60 cells incubated with 131I-IV-14, more than 90% of the radioactivity was detected in the cytosolic fraction (Fig. 4B). The incorporation rate in RNA was about 5%. The associated radioactivity in DNA, protein, and lipid fractions was insignificantly low.
Intracellular Metabolism of IV-14
Pretreatment with 5-fluorouridine initially partially inhibited the metabolism of 131I-IV-14 in Mia-PaCa-2 and HL60 cells. In Mia-PaCa-2 cells (Fig. 4C), about 30% unmetabolized 131I-IV-14 was observed after 2 h of incubation in the 5-fluorouridine–pretreated cells. At this point, only metabolite 1 could be detected in the untreated cells. After 4 h of incubation, only metabolite 1 was observed in 5-fluorouridine–pretreated cells and metabolite 2 was a main metabolite in control cells. After 24 h of incubation, a new metabolite, metabolite 3, together with about 10% metabolite 1 was observed in the 5-fluorouridine–pretreated cells, and mainly metabolite 2 together with some metabolite 3 and metabolite 1 was observed in the untreated cells. All metabolites were completely hydrolyzed into 131I-IV-14 by alkaline phosphatase (Fig. 4D). Retention times of metabolite 1 and nonlabeled IV-14 monophosphate were identical.
Biodistribution of 131I-IV-14 in HL60-Xenografted SCID Mouse Model
131I-IV-14 accumulated at 0.5 h after injection in tumor as well as spleen and small intestine, and in nondividing tissues such as liver, stomach, and kidneys (Fig. 5A). At 4 h after injection, the most retained activity was accumulated in tumor tissue and spleen, whereas the radioactivity in residual normal tissues diminished progressively (Fig. 5B). At 24 h after injection, the radioactivity was almost completely excreted.
Biodistribution of 131I-IV-14 (10 MBq) in HL60-xenografted SCID mice 30 and 60 min after injection (A) and 4 and 24 h after injection (B). Relative account of radioactivity/g in tissue divided by that in tumor (tissue-to-tumor ratio) 30 min after injection: tumor, 1.0 ± 0.15; kidneys, 2.69 ± 1.30; liver, 1.47 ± 0.81; small intestine, 1.48 ± 0.66; spleen, 1.38 ± 0.44. Tissue-to-tumor ratio 4 h after injection: tumor, 1 ± 0.24; spleen, 0.40 ± 0.18; colon, 0.25 ± 0.12; bones, 0.18 ± 0.18; small intestine, 0.14 ± 0.07; others, <0.1. Five animals were used at each time point.
Scintigraphy with 123I-IV-14 in HL60-Xenografted SCID Mouse Model
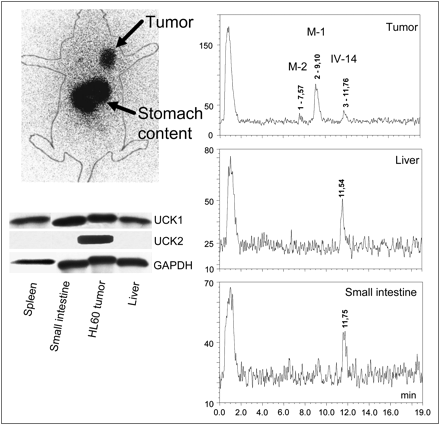
Scintigraphy with 123I-IV-14 (4 h after injection) indicated good tumor visualization with no uptake in unblocked thyroid (Fig. 6). At the same time, high radioactivity enrichment in the content of the stomach and to some extent in the spleen was observed.
At top left is scintigraph obtained with 123I-IV-14 (20 MBq; 4 h) in 3 HL60-xenografted SCID mice. At bottom left is Western blot analysis of UCK1 and UCK2 expression in HL60 xenograft, spleen, small intestine, and liver tissues. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as loading control. At right is analysis of metabolites of 131I-IV-14 (4 h after injection) in HL60-xenografted SCID mice. M-1 = metabolite 1; M-2 = metabolite 2.
Western Blot Analysis of UCK1 and UCK2 in HL60 Xenograft, Spleen, Small Intestine, and Liver
Western blot analysis confirmed the expression of UCK2 in tumor tissue and its absence in normal tissues (spleen, liver, and small intestine). Nearly equal expression of UCK1 was detected in all 4 tested tissues (Fig. 6).
Metabolism of 131I-IV-14 in HL60-Xenografted SCID Mice (4 h After Injection)
Metabolite 1 (70%), metabolite 2 (10%), and IV-14 (20%) were observed in tumor. Only IV-14 was detected in liver and small intestine (Fig. 6). Radioactivity in blood was below the detection limit. Equal quantities of radioiodide and IV-14 were detected in stomach content. Only unchanged IV-14 with traces of 131I− was found in urine (for blood, stomach content, and urine data, see Supplemental Fig. 5).
DISCUSSION
IV-14 is a new ribonucleoside analog of uridine that targets tumor cells expressing UCK2. In the present work, IV-14 showed an UCK2-correlated uptake in a panel of tumors of human origin in vitro and preferential accumulation in UCK2-expressing xenografts in vivo. Thus, IV-14 is a promising candidate as an agent for diagnosis of cancer. The 3′-iodovinyl group allows switching between SPECT, PET, and radiotherapy agents simply by using different iodine radioisotopes (123I, 124I and 131I, or 125I, respectively). Phosphorylation of nucleoside analogs is a prerequisite for the accumulation of IV-14 in tissue. A preferable phosphorylation of a ribonucleoside tracer by tumor-selective UCK2 and not by ubiquitously expressed UCK1 is important for selective enrichment in tumor tissue. The substrate efficiency (Vmax/Km, where Vmax is maximum velocity and Km is the Michaelis constant) of the recombinant UCK2 to 3′-(ethynyl)cytidine and 3′-(ethynyl)uridine was reported to be about 2,000 times higher than that of UCK1 (12). We showed that 131I-IV-14 was taken up mainly by the cell lines with high or moderate expression of UCK2, independently of expression of UCK1. Importantly, virtually no uptake (<0.2%) was detected in the UCK2-negative SK-PC1 cells. The first phosphorylation step catalyzed by UCK is considered rate-limiting (23). Notably, cellular uptake and retention suggested that IV-14 is phosphorylated in these cell lines. HPLC analysis of in vitro metabolites showed transformation of 131I-IV-14 into 3 metabolites with higher polarity than the initial compound. We proposed that phosphorylation of 131I-IV-14 could be a main route for its in vitro metabolism, with metabolites 1, 2, and 3 corresponding to IV-14 mono-, di-, and triphosphates, respectively. Our assumption was supported by complete hydrolysis of all 3 metabolites by alkaline phosphatase, which gave IV-14 as a single product, and by the coincidence of retention times between metabolite 1 and nonlabeled IV-14 monophosphate.
5-Fluorouridine is a substrate for UCK1 and UCK2 with a substrate efficiency (Vmax/Km) about 20-fold higher for UCK2 than for UCK1 (11). Interestingly, after 2 and 4 h of incubation, the cellular uptake and intracellular metabolism of 131I-IV-14 were partially inhibited by 5-fluorouridine.
The biodistribution study with 131I-IV-14 in HL60 SCID mice confirmed the highly selective retention of radioactivity in tumor tissue. The UCK2 specificity of the retention was supported by high level of UCK2 expression in HL60 xenografts and not in normal tissue. Analysis of metabolites supported the role of phosphorylation in selective retention of IV-14 in tumor tissue. IV-14 mono- and diphosphates were detected as main metabolic products in tumor but not in urine, blood, liver, gastrointestinal tract, and gastrointestinal tract contents. Sufficient metabolic stability is essential for successful in vivo application of a radiotracer. A study of structure–activity relationships on several 3′-substituted uridines and cytidines (24) showed that besides 3′-(ethynyl)uridine and 3′-(ethynyl)cytidine, only 3′-propenyluridine and 3′-vinylcytidine could be phosphorylated by UCK at an appreciable rate. On the basis of these data, 3′-iodoethenyluridine should be an apparent choice because of the comparable bulkiness and electronic properties of iodine and a methyl group. However, preliminary experiments showed that radioiodinated iodoethynyl-substituted nucleosides were rapidly deiodinated in human blood serum. Therefore, we synthesized an iodovinyl-substituted nucleoside, IV-14, which demonstrated excellent stability in serum. Furthermore, it was also stable toward human thymidine phosphorylase, which was described to have a broad specificity and contributed to cleavage not only of 2′-deoxyribonucleosides but also of ribonucleosides (25). In contrast, human uridine phosphorylase is highly specific to uridine and is responsible only for 15%−20% cleavage of 5-fluorouridine in blood (26). In contrast to human enzymes, murine uridine phosphorylases play a main role in cleavage of nucleosides in mice (26,27). Appropriate tests showed high stability of IV-14 also toward murine uridine phosphorylases. Surprisingly, in HL60-xenografted SCID mice we observed a high radioactivity concentration in stomach content. Analysis of metabolites showed, along with unchanged IV-14, a significant quantity of radioiodide, indicating deiodination of IV-14 in vivo. This finding was in marked contrast to the good stability of IV-14 toward deiodination in vitro. The mechanism of this deiodination is unclear at present.
Low incorporation of IV-14 in RNA and DNA could explain significant washout of the tracer from the tumor with time. Indeed, nucleoside mono-, di-, and triphosphates could potentially be released via binding cassette transporters and some other transporters as well as during exocytosis (28). It has been also shown that the increased efflux of nucleoside monophosphates via ABCC transporters (ATP-binding cassette transporters, subfamily C)—that is, multidrug resistant proteins 4, 5, and 8—contributes to the resistance of tumor cells against nucleoside drugs (29,30). Nucleoside tri-, di-, and monophosphates could also be hydrolyzed by nucleoside diphosphohydrolases (31) and 5′-nucleosidases (32). Since only intact nucleoside and radioiodide were found in blood, urine, and stomach content, hydrolysis by 5′-nucleosidase followed by efflux via hENT1 transporters could be a more plausible explanation for the washout of IV-14 from the tumor.
hENT1 equilibrating nucleoside transporters play an important role in the intracellular transport of the different nucleosides, such as 3′-deoxy-3′-fluorothymidine, gemcitabine, cytarabine, and fludarabine (33,34). We observed a significant (2.69 ± 0.07 percentage injected dose) accumulation of radioactivity in HL60 cells with moderate UCK2 expression but with a high hENT1 level after 24 h of incubation. The significance of hENT1 for intracellular transport of IV-14 was supported in the case of HL60 cells by a strong inhibition of cellular uptake with p-nitrobenzylmercaptopurine, a known inhibitor of hENT1. However, the only moderate inhibition of cellular uptake of IV-14 with p-nitrobenzylmercaptopurine observed for Mia-PaCa-2 cells suggests possible alternative mechanisms for the intracellular transport of IV-14 in different cell lines.
CONCLUSION
In this study, we showed that cellular retention of IV-14 is strongly dependent on UCK2 expression and its activity, since the protein level correlates with the enzymatic activity (10). A new uridine analog, IV-14, is also selectively taken up by UCK2-expressing HL60 tumor tissue, indicating potential for imaging UCK2-positive tumors in vivo.
Acknowledgments
Prof. Stephan Hahn (Bochum, Germany) is gratefully acknowledged for a generous gift of BX-PC-3, Mia-PaCa-2, Panc-1, and SK-PC-1 cells. We also thank Dr. Annette Sommer (Bayer-Schering, Berlin, Germany) for data on messenger RNA expression of UCK2, Profs. Hans-Jürgen Machulla (Tübingen, Germany) and Gerhard Glatting for critical reading of the manuscript, and Manuela Pape and Ehab Al Mamoni for excellent technical assistance. This work was supported in part by the European Community's FP6 funding (MolDiag-PaCa). This publication reflects only the authors' views. The European Community is not liable for any use that may be made of the information herein.
Footnotes
-
↵* Contributed equally to this work.
-
COPYRIGHT © 2009 by the Society of Nuclear Medicine, Inc.
References
- Received for publication April 29, 2009.
- Accepted for publication July 22, 2009.