Abstract
In both diabetic and nondiabetic patients, there is a loose correlation between coronary flow reserve (CFR) and sympathetic innervation in viable myocardial segments. The loose correlation implies that sympathetic innervation may be preserved even with major impairment of myocardial blood supply. In some patients, denervation is due to repetitive episodes of ischemia in areas with severely reduced CFR. We investigated the long-term effect of reduced CFR on myocardial sympathetic innervation in diabetic and nondiabetic patients with spinal cord stimulation. Methods: We analyzed 23 patients (10 diabetic and 13 nondiabetic) with coronary artery disease and without known cardiac autonomic neuropathy. At baseline, we determined quantitative myocardial blood flow using 13N-ammonia PET, myocardial viability using 18F-FDG PET, and cardiac innervation using 11C-hydroxyephedrine (HED) PET. At the 1-y follow-up we measured CFR and 11C-HED retention. During follow-up, no cardiac intervention was performed and no myocardial infarction occurred. In all patients, spinal cord stimulation was performed for relief of angina. Results: There was no significant difference in segmental 11C-HED retention between baseline and follow-up in the whole patient group. In diabetic patients, as well as in segments with severely reduced CFR (<1.5), 11C-HED retention showed a small but significant decrease (P < 0.05). Linear regression of segmental 11C-HED retention between baseline and follow-up was high (r2 = 0.81), confirming good reproducibility of the investigation on the one hand and little change in regional sympathetic innervation on the other hand. Conclusion: In patients with stable chronic coronary artery disease, sympathetic innervation of the myocardium is almost unchanged in both diabetic and nondiabetic patients in a 1-y follow-up. In myocardial segments with severely altered blood supply, a small but significant decrease in 11C-HED retention most probably reflects ischemic neuronal damage. The prognostic relevance of sympathetic denervation in viable myocardium still has to be determined.
In both diabetic and nondiabetic patients, there is a loose correlation between coronary flow reserve (CFR) and sympathetic innervation in viable myocardial segments (1,2). The loose correlation implies that sympathetic innervation may be preserved even with major impairment of myocardial blood supply. In some patients, denervation is due to repetitive episodes of ischemia in areas with severely reduced CFR.
We investigated the long-term effect of reduced CFR on myocardial sympathetic innervation in diabetic and nondiabetic patients with spinal cord stimulation (SCS) for refractory angina pectoris. During the follow-up, all patients were stable on medication and none underwent a coronary intervention.
The effectiveness of SCS in relieving symptoms in patients with coronary artery disease (CAD) has been shown (3,4). Numerous studies have demonstrated the efficacy of SCS in improving exercise tolerance, decreasing the frequency of anginal episodes, and prolonging the time to electrocardiographic signs of ischemia (5). There is evidence that the pain modulation is due to an inhibition of nociceptive unmyelinated fiber afferents by non-nociceptive myelinated fiber afferents (“gate control theory”) (6,7). Redistribution of myocardial blood flow or even an increase in myocardial blood flow has been discussed as well (8,9). Because it is still an open question whether SCS influences myocardial blood flow, we measured CFR at the follow-up investigation as well.
MATERIALS AND METHODS
Patient Population
We analyzed 23 patients (2 women and 21 men; mean age ± SD, 64 ± 9 y) with chronic CAD. Ten of the patients had type 2 diabetes. In 3 patients, diabetic disease was newly diagnosed. In the remaining 7 patients, the duration of known diabetic disease was 12.1 ± 11.4 y. Glycosylated hemoglobin was 6.9% ± 0.9%, confirming good glycemic control in most patients. Baseline PET was performed to verify the indication for SCS in patients with angina pectoris refractory to medical treatment and without the option of coronary intervention. All patients had angina pectoris, CCS score III or IV (classification of the Canadian Cardiovascular Society) (10). Most patients had 3-vessel disease (22/23), 1 patient, single-vessel disease. Sixteen (70%) of 23 patients had a history of myocardial infarction. Left ventricular ejection fraction measured by echocardiography was 49.7% ± 6.3% in the nondiabetic patients and 43.4% ± 10.7% in the diabetic patients. Patient characteristics are summarized in Table 1.
Patient Characteristics
Patient Investigations
At baseline, 4 PET investigations were performed on each patient. All scans were performed using an ECAT EXACT HR+ PET scanner (CTI/Siemens Medical Systems). Quantitative myocardial blood flow was determined using 13N-ammonia PET. For viability testing, 18F-FDG PET was performed. 11C-hydroxyephedrine (HED) PET was performed for cardiac innervation imaging.
Patients were allowed to continue their medication. Only β-blocking agents were withdrawn, 24 h before 11C-HED PET. The medication consisted of β-adrenoblockers (n = 20), angiotensin-converting enzyme inhibitors (n = 19), long-acting nitrates (n = 20), diuretics (n = 15), statins (n = 21), and antiplatelet drugs (n = 20). All patients took short-acting nitrates if an anginal attack occurred. The patients were asked to abstain from caffeine-containing food or medication 24 h before the stress investigation.
At the 1-y follow-up, 11C-HED PET and quantitative measurement of myocardial perfusion were performed using the same investigation protocols and the same analyses. To improve the comparability between the PET studies with the different tracers on the one hand and between the baseline and the follow-up study on the other hand, the same software was used for the definition of the polar maps in all studies. There was no change in the medication of the patients during follow-up. No cardiac intervention was performed, and no myocardial infarction occurred.
PET
Quantification of Myocardial Blood Flow.
Segmented attenuation correction was performed on the basis of a 5-min transmission scan (68Ge/68Ga rod source) before tracer application. PET scans were acquired dynamically (12 × 10 s, 5 × 30 s, 2 × 120 s, 1 × 450 s) after a bolus injection of 500–600 MBq of 13N-ammonia under rest and stress conditions. At rest, heart rate and blood pressure were measured. Mean arterial blood pressure (systolic plus diastolic divided by 2) was documented, as well as the rate–pressure product (product of heart rate and systolic blood pressure divided by 100). Pharmacologic stress was performed with adenosine (Adenoscan; Sanofi-Aventis GmbH) (0.14 mg/kg/min) over 6 min. Blood pressure (mm Hg) was monitored during adenosine infusion at 1, 3, and 5 min after the beginning of the infusion. Transaxial images were reconstructed iteratively using 4 iterations with 8 subsets of the ordered-subset expectation maximization algorithm). Dynamic area-conserving polar maps were generated using the software created by van den Hoff et al. (11). Quantification of the ammonia studies was based on the irreversible 2-tissue-compartment model described by Hutchins et al. (12). An averaged metabolite correction of the input function was performed on the basis of the results of Rosenspire et al. (13). CFR was calculated by dividing segmental stress perfusion values by segmental rest perfusion values. Coronary resistance was calculated by dividing the mean arterial blood pressure, averaged from the 3 measurements during adenosine infusion, by the segmental stress perfusion value.
Viability Testing.
All patients underwent euglycemic–hyperinsulinemic clamping in preparation for PET. An infusion of 2.3 IU of insulin and 2 mmol of potassium chloride solution in 100 mL of 10% glucose was given over 1 h. In patients with a blood glucose level greater than 160 mg/dL, additional insulin was given intravenously depending on the individual blood glucose level. Halfway through the clamping, 350–400 MBq of 18F-FDG were injected. Acquisition began 30 min after injection. A transmission scan of 10 min was followed by a static emission scan of 15 min. Area-conserving polar maps of relative 18F-FDG uptake were generated using the same software as for the perfusion studies.
Cardiac Sympathetic Innervation.
After a transmission scan of 10 min, 750–800 MBq of 11C-HED were injected as a slow bolus over 30 s. Dynamic PET acquisition was initiated simultaneously (6 × 30 s, 2 × 60 s, 2 × 150 s, 2 × 300 s, 2 × 600 s, 1 × 1,200 s). Image reconstruction was performed as described above. 11C-HED retention (%/min) was calculated by dividing the mean segment activity (kBq/mL) of the last frame over the integrated arterial activity (kBq/mL/min) derived from the ventricular blood pool (14,15).
Statistical Analysis
The left ventricular myocardium was divided into 20 segments (16). For all PET studies, polar maps were generated using the same software to prevent systematic errors caused by different algorithms.
18F-FDG uptake values were normalized individually to the segment with the highest rest perfusion value. In all patients, rest perfusion in the reference segment was normal, so it can be assumed that viability in the reference segments was preserved. A cutoff of 0.7 for normalized 18F-FDG uptake was chosen to define scarred segments. This cutoff was applied because beyond this value there is no more correlation between 11C-HED retention and normalized 18F-FDG uptake (1). Most analyses were performed for viable segments only. Just 11C-HED retention was compared both in all segments and in viable segments only.
A paired t test was performed to compare 11C-HED retention, PET perfusion values, CFR, and coronary resistance in the baseline and follow-up studies. Linear regression analysis was used to test for systematic deviations between baseline and follow-up. Furthermore, myocardial segments were subdivided into 4 groups based on CFR values. These groups consisted of segments with CFR that was normal (≥3), mildly reduced (2 ≤ x < 3), moderately reduced (1.5 ≤ x < 2), and severely reduced (<1.5). A paired t test was performed to compare the 11C-HED retention at baseline and follow-up in the 4 groups. Statistical analysis was performed using StatView 5.0 (SAS Institute Inc.).
RESULTS
Comparison of Baseline Study and Follow-up Study in Whole Collective
There was no significant change in mean arterial blood pressure at rest, rate–pressure product at rest, heart rate, or mean arterial blood pressure during adenosine infusion between baseline and follow-up (Table 2).
Hemodynamic Parameters at Baseline and Follow-up
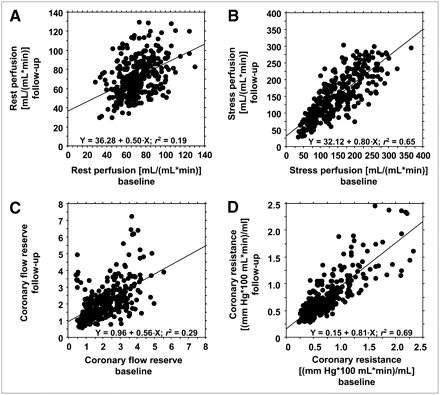
Values for 11C-HED retention, flow values, and coronary resistance in the viable segments are given in Table 3. There was no statistically significant difference in any of the parameters given. Rest perfusion values showed the largest variation between baseline and follow-up (Fig. 1A). Among the different perfusion parameters, coronary resistance showed the highest consistency between the studies (Figs. 1B–1D).
Linear regression of segmental rest perfusion (A), stress perfusion (B), CFR (C), and coronary resistance (D) in nonscarred segments of all 23 patients at baseline (x-axis) and follow-up (y-axis).
Parameters for Viable Segments of All 23 Patients
11C-HED retention agreed well between the baseline and follow-up studies. An example is given in Figure 2. The correlation coefficient was a little bit higher if all segments were included (Fig. 3A). For the nonscarred segments, the correlation coefficient for the 11C-HED retention between the studies was 0.78.
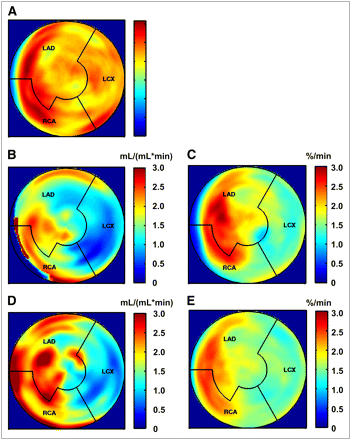
A 75-y-old patient with 3-vessel disease. Bypass graft surgery had been performed 9 y before baseline study; actual coronary angiography revealed dysfunction of graft supplying marginal branch. (A) 18F-FDG PET at baseline showed no sign of myocardial infarction. (B) Stress perfusion at baseline revealed severe perfusion defect in mid anterior and lateral walls. (C) 11C-HED PET at same time point showed diminished 11C-HED retention in segments with diminished stress perfusion. (D and E) One year later, without interim cardiac intervention and after 1 y of SCS, there was little change in myocardial stress perfusion (D) and 11C-HED retention (E).
Linear regression of segmental 11C-HED retention at baseline (x-axis) and follow-up (y-axis) in all segments (A), nonscarred segments (B), nonscarred segments in nondiabetic patients (C), and nonscarred segments in diabetic patients (D).
Comparison of Baseline Study and Follow-up Study in Nondiabetic and Diabetic Patients
Values for 11C-HED retention, flow values, and coronary resistance in the viable segments for the 2 subgroups are given in Table 4. There was a small but significant decrease in 11C-HED retention in the viable segments of the diabetic subgroup. Still, the regression coefficient was a little higher in the diabetic subgroup than in the nondiabetic subgroup (Figs. 3C and 3D). In the nondiabetic patients, significant changes occurred neither in 11C-HED retention nor in the flow parameters. In the diabetic patients, a significant increase in both stress flow and CFR occurred, whereas the increase in coronary resistance was not significant.
Time Course of 11C-HED Retention and Perfusion in Viable Segments of 13 Nondiabetic and 10 Diabetic Patients
Comparison of 11C-HED Retention in Viable Segments at Baseline and Follow-up Classified by CFR
Table 5 shows 11C-HED retention in the viable segments at baseline and follow-up classified by CFR. In segments with severely reduced flow reserve (<1.5), a small but significant decrease in 11C-HED retention took place. In segments with only mildly reduced CFR, there was a small increase in 11C-HED retention.
11C-HED Retention (%/min) in Myocardial Segments Categorized into Groups Based on CFR
DISCUSSION
To our knowledge, this is the first long-term study investigating sympathetic myocardial innervation in patients with stable CAD using 11C-HED PET. The first interesting finding is that the measurement of 11C-HED retention is highly reproducible. Despite the period of 1 y, the correlation coefficient between the studies is high. This high correlation is especially noticeable when one compares the results of the innervation studies with those of the perfusion studies, which show much lower correlation coefficients. The lowest correlation is shown between the rest studies. One possible explanation is that resting myocardial perfusion heterogeneity is a typical feature of CAD itself (17). The natural course of CAD might differ between the patients, too. Even differences in metabolic parameters, such as HDL cholesterol or insulin levels in the plasma, might increase the variability of flow parameters (18,19). The variability of blood flow at rest accounts for the variability of CFR as well. Coronary resistance, which is normalized to arterial blood flow, shows the smallest variability in blood perfusion values.
The 11C-HED retention values in our study are lower than those that other groups have measured in healthy volunteers (14,20,21). One reason for the difference is the methodology used. We calculated 11C-HED retention by dividing the mean myocardial activity of the last frame (40–60 min) by the integrated arterial activity derived from the ventricular blood pool, and we did not correct for metabolites. As shown by Münch et al. (22), use of earlier frames, as well as correction for metabolites, leads to higher values for 11C-HED retention. Another reason for the lower 11C-HED retention is the severe and symptomatic CAD in all our patients.
Aside from the methodologic point of view, the result also shows that there is little change in myocardial sympathetic innervation over time in the absence of myocardial infarctions or coronary interventions. There are only a few exceptions, in which significant changes could be shown. The first exception is 11C-HED retention in viable segments with severely reduced myocardial flow reserve. The small but significant decrease in 11C-HED retention in these segments most probably reflects progressive ischemic neuronal damage (1,2). Still, the difference is apparent only in cases of severe reduction of CFR, not in cases of moderately reduced CFR (Table 4). In segments with only mildly reduced CFR (2 < x < 3), a significant increase in 11C-HED retention was present. A possible explanation might be reinnervation in myocardial segments with improved myocardial perfusion after bypass graft surgery or catheter intervention. We cannot prove this hypothesis, because in all patients interventions had been performed before the baseline study, with different intervals between intervention and study inclusion. Reinnervation has been described in patients after heart transplantation (23,24), but we are not aware of studies showing reinnervation after ischemic neuronal damage.
Another exception is diabetic patients. The mean segmental 11C-HED retention does decrease significantly (Table 3), although the correlation coefficient between the segmental 11C-HED retention at baseline and follow-up is even a little higher than in nondiabetic patients (Fig. 3). One might argue that the reduction in 11C-HED retention is due to progressive autonomic neuropathy (25), but other causes cannot be ruled out, for example, the lower blood flow under stress conditions in the diabetic subgroup (Table 3) or the diminished LV function (Table 1).
Our data have been collected from patients in whom SCS was initiated shortly after the baseline study due to anginal pain refractory to standard treatments. Although we can rule out the possibility that our results have been influenced by myocardial infarctions, coronary interventions, or changes in medication, we cannot rule out that the neurostimulation itself had an impact. The question is whether our results can be applied to other CAD patients, in whom SCS is not performed. The answer to this question is not obvious, mainly because the mechanisms behind the effects of SCS are not completely understood (7,26). The effect of SCS might be due to improvement in myocardial blood flow (8,9,27). Our data do not support this thesis, as blood flow under rest and stress conditions, as well as CFR, did not change significantly (Table 2). Another hypothesis is that SCS reduces sympathetic tone, decreasing myocardial oxygen consumption and improving myocardial microcirculatory blood flow (26,28). We cannot rule out the possibility that the assumed reduction in sympathetic tone leads to a protective effect on the sympathetic neurons themselves, causing the neurons to be less susceptible to ischemic damage. It is difficult to provide a control group of nontreated patients because patients with proven ischemia are usually treated by revascularization or, if coronary intervention is not an option, by alternative approaches.
The prognostic relevance of sympathetic denervation in viable myocardium is still under discussion. There is evidence that the autonomic nervous system plays a major role in the pathogenesis of sudden cardiac death (29–31). Actually under investigation is whether 11C-HED PET is suitable for risk assessment for sudden cardiac death (32). We have shown that sympathetic innervation does change slightly over time, despite symptomatic CAD and severely reduced CFR. Interestingly, percutaneous coronary intervention, when added to optimal medical therapy, did not reduce the risk of death or other major cardiovascular events (33). Both findings might be related in the following sense. The temporary improvement in myocardial blood supply caused by the percutaneous coronary intervention is unlikely to change the innervation pattern of the left ventricular myocardium. Because the innervation pattern itself is a major risk factor for sudden cardiac death, percutaneous coronary intervention might not reduce the risk of death either. The possible interrelationships between revascularization procedures, myocardial sympathetic innervation, and prognosis will be a matter of further investigations.
CONCLUSION
In the absence of cardiac intervention or myocardial infarction, there is little change in sympathetic innervation of the myocardium in either diabetic or nondiabetic patients in a 1-y follow-up. In myocardial segments with severely altered blood flow, a small but significant decrease in 11C-HED retention most probably reflects ischemic neuronal damage. The prognostic relevance of sympathetic denervation in viable myocardium still has to be determined.
Footnotes
-
COPYRIGHT © 2008 by the Society of Nuclear Medicine, Inc.
References
- Received for publication March 3, 2008.
- Accepted for publication May 28, 2008.