Abstract
99mTc-Annexin-V imaging has been proved to be feasible to detect phosphatidylserine, which externalizes on the outer cell membrane early in the process of apoptosis. To determine whether postconditioning suppresses myocardial cell damage or apoptosis, we evaluated the intensity and distribution of 99mTc-annexin-V uptake after postconditioning in a rat model of ischemia and reperfusion and compared the effect to that of ischemic preconditioning and pretreatment with caspase inhibitor. Methods: In control rats (n = 13), after thoracotomy the left coronary artery was occluded for 20 min followed by reperfusion for 30 or 90 min and injection of 99mTc-annexin-V (80–150 MBq). One hour later, to verify the area at risk, 201Tl (0.74 MBq) was injected intravenously just beyond the left coronary artery reocclusion, and the rats were sacrificed 1 min later. In the groups of rats with various interventions, postconditioning (n = 11) was performed just after the reperfusion, and preconditioning (n = 11) and caspase inhibitor treatment (n = 11) were performed before ischemia. Dual-tracer autoradiography was performed to assess 99mTc-annexin-V uptake and area at risk. Results: In all control rats, intense 99mTc-annexin-V uptake was observed in the area at risk (uptake ratios at 30 or 90 min after reperfusion, 4.15 ± 1.89 and 3.70 ± 1.41, respectively). Postconditioning suppressed 99mTc-annexin-V uptake (uptake ratios at 30 or 90 min after reperfusion, 2.09 ± 0.56, P < 0.05, and 1.88 ± 0.69, P < 0.05, respectively). Preconditioning also suppressed uptake (uptake ratios at 30 and 90 min after reperfusion, 1.17 ± 0.29, P < 0.005, and 1.33 ± 0.74, P < 0.01, respectively), as did caspase inhibitor (uptake ratios at 30 and 90 min after reperfusion, 2.08 ± 0.50, P < 0.05, and 1.27 ± 0.24, P < 0.005, respectively). In all interventions, the percentage of cells positive on deoxyuride-5′-triphosphate biotin nick end labeling and histologic changes with myocardial cell degeneration and cell infiltrations were suppressed markedly. Conclusion: These data indicate that 99mTc-annexin-V imaging may be a way to monitor myocardial injury and its response to novel therapeutic interventions including postconditioning, preconditioning, and antiapoptotic therapy.
Annexin-V labeled with 99mTc was developed to image ongoing apoptotic cell death in vivo via specific binding to exposed phosphatidylserine with a high affinity (1–7). In healthy cells, phosphatidylserine is actively transported from the outer to the inner leaflet of the cell membrane by an aminophospholipid translocase. Once cells activate their cell death program, phosphatidylserine from the inner leaflet of the membrane is externalized. This phosphatidylserine expression is an early sign that the cell death program is activated (8,9). Several animal experiments have demonstrated that myocardial ischemia followed by reperfusion results in a substantial loss of myocardial cells through apoptosis. Although there is evidence that the apoptotic pathway can be initiated during ischemia, it appears that induction of apoptosis after brief ischemia is fully executed during reperfusion (10). Our previous study on a rat model with 20 min of ischemia and reperfusion showed that phosphatidylserine externalization commences soon after the ischemia/reperfusion and that 99mTc-annexin-V uptake in the ischemic area peaked 30 min after reperfusion and gradually declined over 3 d (11). These findings imply that myocardial cells in ischemic areas might be rescued if the apoptotic process was efficiently blocked during the early phase of reperfusion. Ischemic preconditioning produced by a prior short interval of coronary occlusion and reperfusion has been demonstrated to result in marked cardioprotection during a subsequent period of sustained ischemia. This protective effect on myocardial injury has been shown to be achieved, in part, by decreasing apoptosis after ischemia and reperfusion.
Zhao et al. (12) demonstrated that brief, intermittent ischemia applied during the onset of reperfusion (postconditioning) is cardioprotective in a canine model of ischemia and reperfusion. However, the effect of postconditioning on apoptosis (13) or 99mTc-annexin-V uptake is unspecified. Therefore, the aim of this study was to determine the effect of postconditioning on the intensity and distribution of 99mTc-annexin-V uptake in a rat model of ischemia and reperfusion and to compare it with that of ischemic preconditioning and pretreatment with caspase inhibitor.
MATERIALS AND METHODS
Animal Model of Acute Ischemia and Reperfusion
All experimental procedures involving animals were conducted in accordance with the institutional guidelines set by the Institute for Experimental Animals, Kanazawa University Advanced Science Research Center. Ten- to 14-wk-old male Wistar rats (n = 46) were anesthetized with an intraperitoneal administration of 40 mg of pentobarbital per kilogram and ventilated mechanically with room air. After left thoracotomy and exposure of the heart, a 7-0 polypropylene suture on a small curved needle was passed through the myocardium beneath the proximal portion of the left coronary artery (LCA), and both ends of the suture were passed through a small vinyl tube to make a snare. The suture material was pulled tightly against the vinyl tube to occlude the LCA. Myocardial ischemia was confirmed by ST-segment elevation on the electrocardiogram and regional cyanosis of the myocardial surface. Four series of experiments were performed (Fig. 1). In control rats (n = 13), the LCA was occluded for 20 min and reperfusion was obtained by release of the snare and confirmed by a myocardial blush over the area at risk. The snare was left loose on the surface of the heart for reocclusion of the LCA just before sacrifice to identify the area at risk. 99mTc-Annexin-V (80–150 MBq) was administered at 30 min (n = 6) or 90 min (n = 7) after reperfusion. One hour after injection of the 99mTc-annexin-V, 0.74 MBq of 201Tl was injected via a tail vein just after reocclusion of the proximal portion of the LCA for delineation of the area at risk. One minute later, the rat was euthanized and the heart was removed for analysis (Fig. 1A). In the group of rats with postconditioning (n = 11), after the 20 min of LCA occlusion, 10 s of reperfusion followed by 10 s of LCA reocclusion was repeated 6 times at the beginning of the 30 min (n = 6) or 90 min (n = 5) of reperfusion (Fig. 1B). In the group of rats with ischemic preconditioning (n = 11), 5 min of LCA occlusion followed by 5 min of reperfusion was repeated 4 times, and a 20-min LCA occlusion was performed. After 30 min (n = 6) or 90 min (n = 5) of reperfusion, the same procedure that was performed on the control rats was fulfilled (Fig. 1C). In the group of rats with caspase inhibitor (n = 11), an 0.85 mg/kg dose of a broad-spectrum caspase inhibitor, quinolyl-valyl-O-methylaspartyl-[-2,6-difluorophenoxy]-methyl ketone (Q-VD-OPh) (Calbiochem) (14), was infused via a tail vein. Twenty minutes later, the LCA was occluded for 20 min. After 30 min (n = 6) or 90 min (n = 5) of reperfusion, the same procedure that was performed on the control rats was fulfilled (Fig. 1D). The excised heart was rinsed in saline, frozen in isopentane, cooled in dry ice, and embedded in methyl cellulose. Serial short-axis heart sections 20 μm thick were obtained by sectioning on a cryostat to create a series of rings for autoradiography.
Experimental protocol. Control rats (A), rats with ischemic postconditioning (B), rats with ischemic preconditioning (C), and rats with caspase inhibitor treatment (D).
Radiolabeling of Annexin-V
Mutant annexin-V (annexin-V-117 mutant, a form of recombinant human annexin engineered to include a binding site for technetium) was prepared through expression in Escherichia coli as previously described (5). This material retains phosphatidylserine binding activity equivalent to that of native annexin-V. A specific activity of 3.7–7.4 MBq (100–200 μCi)/μg of protein with a radiopurity of more than 90% was achieved using the previously described radiolabeling protocol (5).
Dual-Tracer Autoradiography
Dual-tracer autoradiography of the left ventricular short-axis slices was performed to assess 99mTc-annexin-V uptake and ischemic area (201Tl uptake). The first autoradiographic exposure on an imaging plate (BAS-MS; Fuji Film) was performed for 15–20 min to visualize 99mTc-annexin-V distribution 1–2 h after sacrifice. Three days later (12 half-lives of 99mTc), the second exposure was made for 24 h to image the area at risk expressed by 201Tl distribution.
Data Analysis
99mTc-Annexin-V accumulation was evaluated in 3 myocardial slices at the mid-ventricular level spaced 1 mm apart from one another. Distribution of the tracers was determined by analysis of the digitized autoradiographs. The photostimulated luminescence in each pixel (100 × 100 μm) was determined using a bioimaging analyzer (BAS-5000; Fuji Film). For quantitative analysis, the uptake values for each region of interest (ROI) were expressed as the background-corrected photostimulated luminescence per unit area (1 mm2). A background ROI was set adjacent to the left ventricle. Ischemic and normally perfused areas were defined from the 201Tl image, and these ROIs were applied to the 99mTc-annexin-V images to evaluate uptake of 99mTc-annexin-V. An area of significant 99mTc-annexin-V uptake was also defined manually as an ROI. The 99mTc-annexin-V uptake ratio was calculated by dividing the uptake value for an ischemic area by that for a normally perfused area. The ratio of the ROI in an area of 99mTc-annexin-V uptake to the ROI in an area of ischemia was defined as the 99mTc-annexin-V uptake area and expressed as a percentage. All parameters in each rat were expressed as an average value obtained from analysis of 3 representative slices.
In Situ Detection of Nuclear DNA Fragmentation (TUNEL)
In all reperfusion models, short-axis frozen sections adjacent to the slices for autoradiography were mounted on slides. These short-axis heart sections were processed using deoxyuride-5′-triphosphate biotin nick end labeling (TUNEL) staining. The staining was performed with the in situ cell death detection kit, POD, according to the manufacturer's protocol (Roche Diagnostics GmbH). The number of TUNEL-positive cardiomyocytes was divided by the total number of cardiomyocytes to determine the ratio of TUNEL-positive myocytes within the area at risk and the normally perfused area. More than 50 different fields for each section were analyzed. As a positive control, we used rat intestine. Several epithelial cells in the villous tip showed positive staining.
Histopathologic Examinations with Light Microscopy
Light microscopic histopathologic examinations were performed on hematoxylin- and eosin-stained slices obtained from the rats 24 h after reperfusion, because no significant histologic alterations were observed after 30 or 90 min of reperfusion in control rats.
Statistical Analysis
All results were expressed as mean ± 1 SD. Statistical analyses were performed using a Macintosh computer with StatView software, version 5.0. Group comparisons were performed using ANOVA, followed by the Scheffé test to identify differences among groups. A value of P less than 0.05 was considered statistically significant.
RESULTS
Size of Area with 99mTc-Annexin-V Uptake Against Area at Risk
In rats with preconditioning, 3 of 6 rats after 30 min of reperfusion and 1 of 5 rats after 90 min of reperfusion showed no significant 99mTc-annexin-V uptake visually; therefore, the 99mTc-annexin-V uptake area was calculated only in the rats with visually identifiable 99mTc-annexin-V uptake.
The 99mTc-annexin-V uptake area in rats after 30 min of reperfusion with postconditioning, preconditioning, and Q-VD-OPh was similar to that in control rats (52.0% ± 9.0%, 64.4% ± 7.2%, 59.6% ± 3.9%, respectively, vs. 51.3% ± 16.2%; P = not statistically significant).
In rats after 90 min of reperfusion, the 99mTc-annexin-V uptake area in rats with postconditioning and Q-VD-OPh was also similar to that in control rats (56.6% ± 3.1% and 52.9% ± 5.4%, respectively, vs. 60.2% ± 3.5%; P = not statistically significant). In rats with preconditioning, the 99mTc-annexin-V uptake area (43.7% ± 7.7%) was smaller than that in control rats (P < 0.001) or that with postconditioning (P < 0.01).
99mTc-Annexin-V Uptake
On visual analysis, significant 99mTc-annexin-V uptake was observed predominantly at the mid myocardium in the area at risk in control rats after 30 or 90 min of reperfusion (Fig. 2). Ischemic postconditioning markedly attenuated uptake of 99mTc-annexin-V in rats with either 30 or 90 min of reperfusion. In preconditioning, 99mTc-annexin-V uptake was almost imperceptible in rats with either 30 or 90 min of reperfusion. Caspase inhibitor treatment apparently reduced 99mTc-annexin-V uptake at 30 min after reperfusion and suppressed uptake nearly completely at 90 min after reperfusion (Fig. 2).
Autoradiography of 99mTc-annexin-V and 201Tl. After 20 min of ischemia, 99mTc-annexin-V was injected at 30 or 90 min after reperfusion. Single mid-ventricular slices are shown from representative animals from each group. 201Tl images demonstrate area at risk. Intense 99mTc-annexin-V uptake is observed in area at risk in control rat; however, uptake was markedly decreased by postconditioning and preconditioning and by caspase inhibitor treatment.
On quantitative analysis, the 99mTc-annexin-V uptake ratios of control rats at 30 or 90 min after reperfusion were 4.15 ± 1.89 and 3.70 ± 1.41, respectively. The 99mTc-annexin-V uptake ratio at 30 or 90 min after reperfusion was significantly suppressed by postconditioning (2.09 ± 0.56, P < 0.05, and 1.88 ± 0.69, P < 0.05, respectively). Ischemic preconditioning also suppressed 99mTc-annexin-V uptake markedly after 30 or 90 min of reperfusion (1.17 ± 0.29, P < 0.005, and 1.33 ± 0.74, P < 0.01, respectively). Caspase inhibitor pretreatment attenuated 99mTc-annexin-V uptake significantly at 30 min after reperfusion (2.08 ± 0.50, P < 0.05) and at 90 min after reperfusion (1.27 ± 0.24, P < 0.005) (Fig. 3). When the 3 groups of interventions were compared in terms of 99mTc-annexin-V uptake ratio, preconditioning showed significantly less uptake than postconditioning (P < 0.05) or caspase inhibitor treatment (P < 0.05) at only 30 min after reperfusion.
99mTc-Annexin-V uptake ratios in control rats (cont) and in rats with postconditioning (poscon), preconditioning (precon), and caspase inhibitor. 99mTc-Annexin-V uptake ratio was calculated by dividing 99mTc-annexin-V count density in area at risk by that in nonischemic area. Reperfusion time indicates time of 99mTc-annexin-V injection after reperfusion. Compared with control rats, all 3 interventional groups demonstrated significant reduction of 99mTc-annexin-V uptake.
TUNEL-Positive Cardiomyocytes
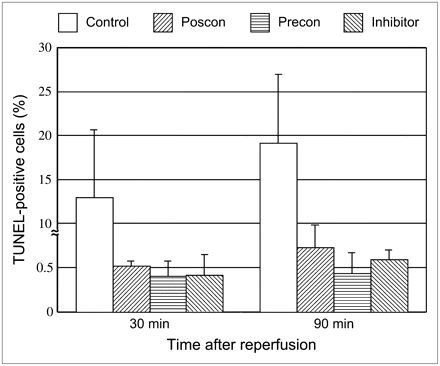
The percentage of TUNEL-positive cells in ischemic areas at 30 or 90 min after reperfusion in the rats with 20 min of ischemia was 15.1% ± 5.6% and 19.2% ± 7.7%, respectively. Postconditioning significantly attenuated the TUNEL-positive cells in ischemic areas in both the 30- and the 90-min reperfusion models (0.52 ± 0.055, P < 0.0001, and 0.73% ± 0.25%, P < 0.0001, respectively). The percentage of TUNEL-positive cells in ischemic areas at 30 or 90 min after reperfusion was also markedly suppressed by ischemic preconditioning (0.40% ± 0.23%, P < 0.0001, and 0.44% ± 0.23%, P < 0.0001, respectively) and by caspase inhibitor (0.42% ± 0.23%, P < 0.0001, and 0.59% ± 0.11%, P < 0.0001, respectively) (Figs. 4 and 5).
Percentage of TUNEL-positive cardiomyocytes in control rats (cont) and in rats with postconditioning (poscon), preconditioning (precon), and caspase inhibitor. Myocardial percentage of TUNEL-positive staining is markedly depressed by postconditioning, preconditioning, and caspase inhibitor, compared with control rats (P < 0.0001).
TUNEL staining. (A) Control rat. Several cardiomyocytes have become TUNEL-positive (brown staining of nucleus) after ischemia and 90 min of reperfusion. (B) Postconditioning rat. Small number of cardiomyocytes showed TUNEL-positive staining after ischemia and 90 min of reperfusion. (C) Preconditioning rat. Minimal numbers of cardiomyocytes demonstrated TUNEL-positive staining after ischemia and 90 min of reperfusion. (D) Rat with caspase inhibitor. Minimal numbers of cardiomyocytes demonstrated TUNEL-positive staining after ischemia and 90 min of reperfusion.
The percentage of TUNEL-positive cells in nonischemic areas at 30 or 90 min after reperfusion was 0.073% ± 0.011% and 0.071% ± 0.055%, respectively, in control rats, 0.052% ± 0.014% and 0.060% ± 0.022%, respectively, in postconditioning rats, 0.065% ± 0.022% and 0.049% ± 0.009%, respectively, in preconditioning rats, and 0.049% ± 0.013% and 0.052% ± 0.014%, respectively, in caspase inhibitor rats.
Histopathologic Findings
In the control rats, light microscopic examination of the hematoxylin- and eosin-stained slices from frozen specimens at 24 h after reperfusion revealed hypereosinophilic cardiac muscle fibers due to coagulation necrosis, muscle lysis, and muscle destruction. Extensive neutrophilic infiltrates were also observed between the fibers. In contrast, the rats with postconditioning showed no neutrophilic infiltrations but a few foci of muscle lysis with enlarged, aggregated myocardial nuclei at 24 h after reperfusion. However, the area of these changes was less than 1% of each ischemic area. The rats with preconditioning and caspase inhibitor also showed microfoci of muscle lysis and enlarged, aggregated myocardial nuclei but no neutrophilic infiltration. The area of these changes was also less than 1% of each ischemic area.
DISCUSSION
Postconditioning was originally discovered and described by Zhao et al. (12,13) as the reduction of infarct size by several cycles of coronary occlusion and reperfusion after sustained ischemia. The most effective treatment to reduce infarct size resulting from myocardial ischemia is the restoration of blood flow by reperfusion. However, reperfusion has the potential to induce additional injury that is not evident at the end of ischemia, so-called reperfusion injury. Postconditioning seems to attenuate many manifestations of reperfusion such as endothelial activation and dysfunction, infarction, and apoptosis.
The present study demonstrated that ischemic postconditioning significantly suppressed 99mTc-annexin-V uptake during the early phase of ischemia and reperfusion in conjunction with a marked reduction in the number of myocardial TUNEL-positive cells. Light microscopic examination also showed marked improvement in myocardial tissue injury after postconditioning, with only minimal cell infiltrates and focal muscle lysis after 24 h of reperfusion. These data suggest that ischemic postconditioning reduces apoptosis as detected by a reduction of externalization of phosphatidylserine and DNA fragmentation (TUNEL staining) due to myocardial ischemia and reperfusion.
Caspase inhibitor treatment also similarly attenuated 99mTc-annexin-V uptake, the rate of TUNEL-positive cells, and histologic changes. Ischemic preconditioning suppressed 99mTc-annexin-V uptake more completely. In all groups of interventions, the percentage of TUNEL-positive cells declined dramatically after interventions, though the percentage of TUNEL-positive cells in ischemic areas was 6–12 times higher than that in nonischemic areas.
Ischemic preconditioning is achieved by brief episodes of ischemia and reperfusion before prolonged coronary artery occlusion; in contrast, postconditioning is triggered by rapid intermittent interruptions of blood flow just after relief of prolonged ischemia. The periods of reperfusion and ischemia in postconditioning are rather short in smaller animals (10–15 s in rats and mice) and are longer in larger animals and in humans (30 s in rabbits and canines and 60 s in humans) (12,15–18). In rat heart in vivo, postconditioning attenuated the production of reactive oxygen species just after reperfusion, and the cardioprotective effect of the postconditioning was lost if instituted after 1 min of reperfusion (16). In contrast to preconditioning, an experimental protocol of postconditioning appears to be applicable to patients with ongoing acute myocardial infarction who undergo coronary intervention, because postconditioning is initiated at the time of reperfusion and has proved to be feasible in a clinical trial (18). To patients who underwent coronary angioplasty for ongoing myocardial infarction, Staat et al. (18) applied postconditioning within 1 min of reflow by 4 episodes of 1 min of inflation and 1 min of deflation of the angioplastic balloon. The authors found that the area under the curve of creatine kinase release was significantly less (a 36% reduction) in the postconditioning group than in the control group and concluded that postconditioning by coronary angioplasty protects the human heart during acute myocardial infarction.
Postconditioning has been proven to reduce myocardial necrotic death by activating survival kinases, and it has been suggested that both preconditioning and postconditioning may share a common pathway as a mechanism of their cardioprotective effect (17–19). In addition to necrosis, Wang et al. (20) showed that hypoxic postconditioning after a prolonged period of hypoxia of isolated neonatal cardiomyocytes attenuated the apoptosis detected by the reduction of TUNEL-positive cells and DNA ladders. Using a similar experimental system, Sun et al. (21). also demonstrated that hypoxic postconditioning reduced the number of TUNEL-positive cells and caspase-3 activity. The results in the present study were consistent with those of this previous study and demonstrated for, what is to our knowledge, the first time in an in vivo rat model of ischemia and reperfusion that postconditioning attenuates myocardial apoptosis by detecting the reduction of 99mTc-annexin-V uptake and TUNEL-positive cells. Compared with preconditioning, inhibition of 99mTc-annexin-V uptake in postconditioning was somewhat less complete. In postconditioning, 99mTc-annexin-V uptake was suppressed by 70% of the control (when uptake ratio 1 was considered as 100% suppression); however, in preconditioning, 99mTc-annexin-V uptake was suppressed by more than 90%. This finding is consistent with the finding that the reduction of infarct size by postconditioning is less marked than that by preconditioning in rats and rabbits (16,17).
In general, cell death is classified as a highly regulated energy-consuming process (apoptosis) or a non–energy-consuming process (oncosis/necrosis). However, a previous study revealed that the morphology of annexin-V–positive myocardial cells after ischemia and reperfusion showed characteristics of both apoptosis and oncosis (22), suggesting that the process of apoptosis might not be identical in a complex and large cell, such as the myocardial cell, to that originally described by Kerr et al. (23). Myocardial uptake of 99mTc-annexin-V in this study is believed to result from 99mTc-annexin-V binding to the externalized phosphatidylserine, in view of the results of Dumont et al., who found that a high prevalence of annexin-V–positive cardiomyocytes after ischemia and 30 or 90 min of reperfusion accompanied minimal IgG staining, a marker of cell membrane leakage. Also, our previous study suggested that 99mTc-annexin-V uptake early after reperfusion reflected phosphatidylserine externalization because 99mTc-annexin-V uptake was highest at 30 min after reperfusion without histologic changes, and the uptake decreased to less than half at 24 h after reperfusion, when histology showed a necrotic morphology. In addition, generalized caspase inhibitor suppressed 99mTc-annexin-V uptake significantly, indicating that most of the uptake of 99mTc-annexin-V reflects apoptotic activation.
CONCLUSION
The present study demonstrated that 99mTc-annexin-V uptake after myocardial ischemia and reperfusion is significantly suppressed by postconditioning along with a marked reduction in the number of TUNEL-positive cells and negligible or no necrosis. A comparable reduction of 99mTc-annexin-V uptake was obtained by pretreatment with generalized caspase inhibitor. Ischemic preconditioning suppressed 99mTc-annexin-V uptake more markedly. These data indicate that 99mTc-annexin-V imaging may be a novel way to monitor myocardial injury and its response to novel therapeutic interventions, including postconditioning, preconditioning, and antiapoptotic therapy.
Acknowledgments
We thank Dr. Jean-Luc Vanderheyden for helpful discussions and a critique of the manuscript. This study was supported in part by grants-in-aid for scientific research (C-14570842 and C-17591253) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Footnotes
-
COPYRIGHT © 2007 by the Society of Nuclear Medicine, Inc.
References
- Received for publication January 29, 2007.
- Accepted for publication May 7, 2007.