Abstract
The aim of this study was to image the extra domain B (ED-B) of fibronectin, an angiogenesis-related target, in solid tumors using small-animal PET. Toward this aim, an ED-B fibronectin-binding human antibody derivative (L19-SIP) was labeled with 76Br via an enzymatic approach. Biodistribution and imaging studies were performed in human teratoma–bearing mice for up to 48 h after injection. Methods: L19-SIP was labeled with 76Br using bromoperoxidase/H2O2. The stability of the labeled antibody was tested both in vitro and in vivo. Biodistribution and small-animal imaging studies (PET and CT) were performed in F9-bearing 129/sv mice (n = 3 or 4). Results: The enzymatic radiobromination approach afforded the labeled antibody in high yield (>55%) under mild reaction conditions. 76Br-L19-SIP stability in mouse serum proved to be similar to that of the 125I-labeled analog (>80% of intact material at 48 h after injection). Fast and specific in vivo targeting was obtained in tumors and other organs expressing ED-B fibronectin (i.e., ovaries and uterus). However, slow renal clearance and persistent activity predominately in blood and stomach suggests partial 76Br-L19-SIP debromination in vivo. This debromination was confirmed in a metabolism study in normal mice. The F9 tumors were clearly imaged by small-animal PET at each considered time point, starting at 5 h up to 48 h after injection. Conclusion: 76Br-L19-SIP specifically accumulated at the target site, enabling detailed small-animal PET of tumor neovasculature. Therefore, targeting the angiogenesis-associated expression of ED-B fibronectin can be a valuable tool for tumor detection using molecular imaging with PET.
A key parameter for tumorigenesis and rapid tumor growth is a strong vascularization. Access to the host vascular system and the generation of new blood vessels are, therefore, rate-determining steps in tumor growth. Small, dormant tumors (1–2 mm) rely on oxygen and nutrient diffusion from the surrounding tissues to survive (avascular phase). Tumor progression and metastasis propagation occur only after induction of a tumor vasculature (angiogenic switch) (1). Therefore, angiogenic activity is an interesting target for a specific personalized tumor therapy. Currently, many new potential antiangiogenic agents are under evaluation in clinical trials. A reliable patient selection as well as an early therapy- monitoring tool will be a prerequisite for an efficient therapy development and patient management (2). The first PET studies in prostate cancer patients undergoing antiangiogenic therapy have been performed by monitoring changes in tumor blood flow (15O-water), blood volume (11CO), and tumor metabolism (18F-FDG) (3). 11C-Methionine uptake has also been suggested as a surrogate marker for an indirect measure of tumor angiogenesis (4). However, more direct visualization of tumor vasculature and better evaluation of changes after antiangiogenic therapy can be obtained by targeting specific proteins involved in the angiogenic process. Among these, the vascular endothelial growth factor receptor (VEGFR) (5–7) and the αvβ3 integrin have been extensively evaluated for PET of angiogenesis (8–14).
The fibronectin splice variant B (ED-B) is another specific biomarker of angiogenesis (15). In fact, it is expressed around the neovasculature of a variety of human cancers, both in primary and metastatic sites (16,17) as well as in fetal tissues and in the female reproductive system, where tissue remodeling and angiogenesis are ongoing or recurrent processes. Therefore, imaging the expression of ED-B fibronectin may be a very useful tool to assess the efficacy of antiangiogenic therapies at an early stage.
Recently, a human recombinant scFv fragment (L19) has been developed with subnanomolar binding affinity for ED-B of fibronectin (18). In a preliminary clinical study, liver metastases of colon carcinoma, small cell lung cancer, and recurrent glioblastoma were imaged using an 123I-labeled L19(scFv)2 (19). After that, several L19 formats were developed and evaluated in vivo for both radioimmunotherapy (20–23) and imaging (24). Among these, the L19 small immunoprotein (SIP) demonstrated the best performance in vivo in terms of tumor targeting and clearance from nontarget organs (20,25).
Driven by these promising results, we decided to explore the use of L19-SIP for PET of tumor neovasculature as this could be a particularly attractive imaging approach, providing both high spatial-resolution images and quantitative information on tracer distribution. Because of the biologic half-life of L19-SIP, a positron-emitting radionuclide with a half-life longer than that of 18F (109 min) was needed for PET of ED-B fibronectin. Among the radiohalogens, the positron emitters 76Br (half-life [t1/2] = 16.2 h) and 124I (t1/2 = 4.18 d) are under investigation for the production of PET radiotracers. There is considerable interest in 76Br labeling of antibodies (26–31) because of its favorable half-life that allows imaging up to 48 h after injection, its high production yields (at least an order of magnitude higher than that of 124I), and its 54% positron emission (∼2-fold higher than that of 124I) (32). For these reasons, and due to the well-established labeling chemistry, we have labeled L19-SIP with 76Br. Small-animal PET and biodistribution studies were performed to evaluate the potential of such a PET probe. The results clearly indicated ED-B fibronectin targeting in vivo, thus confirming the possible use of L19-SIP to image angiogenesis with PET.
MATERIALS AND METHODS
Reagents
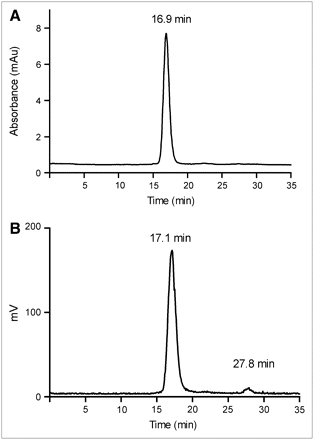
Unless otherwise listed, all solvents and reagents were purchased from Sigma-Aldrich and used as received. NAP-5 columns were purchased from GE Healthcare Biosciences. Water was distilled and then deionized (18 MΩ/cm2) by passing through a Milli-Q water filtration system (Millipore Corp.). L19-SIP was expressed and purified as described earlier (25). 76Br was produced at the Washington University cyclotron facility by the 76Se(p,n)76Br nuclear reaction on a 76Se-enriched Cu2Se target. 76Br was recovered via a dry distillation method modified from that of Tolmachev et al. (33). The radionuclide solution (in 0.6 mol/L NH4OH) was filtered through a C-18 Sep-Pak light cartridge (Waters Corp.) and blown down to dryness. The radioactive bromide was reconstituted in the radiolabeling buffer shortly before use. 125I-Iodide was purchased from GE Healthcare Biosciences. Fast-protein liquid chromatography (FPLC) and radio-FPLC were performed using an Amersham Pharmacia Biotech ÄKTA FPLC system (GE Healthcare Biosciences) equipped with a model 170 Radioisotope Detector (Beckman Instruments). FPLC analysis was performed by injecting a 2-μL analyte aliquot into a Superose 12 gel filtration column (GE Healthcare Biosciences), which was eluted with 20 mmol/L N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) (HEPES) and 150 mmol/L NaCl buffer (pH 7.3) at an isocratic flow rate of 0.8 mL/min. The ultraviolet wavelength was preset at 280 nm. Under these conditions, the retention times of (76Br)L19-SIP and 76Br-bromide were 16.9–17.1 min and 27.8 min, respectively.
L19-SIP Radiolabeling
The 76Br-bromination protocol was modified from that of Lovqvist et al. (28). One hundred micrograms of L19-SIP were mixed with ∼25–90 MBq (∼0.67–2.43 mCi) 76Br and 0.6 unit bromoperoxidase (BPO) in 300 μL 50 mmol/L phosphate buffer (pH 7.0) containing 80 μmol/L H2O2. The reaction mixture was incubated at 0°C and monitored by radio-FPLC. The radiobrominated antibody was purified by chromatography using a NAP-5 column eluted with 0.8 mL phosphate-buffered saline (PBS) and then analyzed by radio-FPLC. Samples having >90% radiochemical purity (RCP) were further diluted with PBS and used for animal studies. The immunoreactivity of 76Br-L19-SIP was determined by affinity chromatography as previously reported (20,24). The radioiodination (125I) of L19-SIP was performed as previously reported (20,24). The in vitro stability of the 76Br-L19-SIP and 125I-L19-SIP was evaluated by incubating duplicate samples of the compound in mouse serum (Sigma-Aldrich) at 37°C and by analyzing aliquots (filtered through 0.45 μm) by radio-FPLC at different time points up to 48 h.
Cell Lines and Animals
Mouse embryonal teratocarcinoma cells (F9), obtained from American Type Culture Collection, were cultivated in Dulbecco's modified Eagle medium with Glutamax (Invitrogen Corp.) supplemented with 10% (v/v) fetal calf serum and maintained at 37°C in a humidified atmosphere containing 5% CO2. Four-week-old female 129/sv mice (Charles River Laboratories) were injected with 1 × 106 F9 cells in 100 μL PBS subcutaneously into the right hindlimb. After 11 d, the animals were used for biodistribution and imaging experiments (tumor weight, 0.1–2.8 g). All animal studies were performed in compliance with guidelines set by the Washington University Animal Studies Committee.
Biodistribution Studies
The F9 tumor-bearing mice (n = 3 or 4 per time point) were anesthetized with isoflurane and injected intravenously with ∼1.3 MBq of 76Br-L19-SIP (∼185 kBq/μg) in 150 μL via the femoral vein. At 5, 24, and 48 h after injection, the mice were anesthetized and sacrificed by cervical dislocation. Organs and tissues of interest were removed, blotted dry, weighed, and counted. Diluted standard doses (1:100) were prepared and counted along with the samples to calculate the percentage injected dose per gram (%ID/g) and the percentage injected dose per organ (%ID/organ). All data were corrected for radioactive decay of 76Br.
Small-Animal PET Studies
F9 tumor-bearing mice (n = 4) were anesthetized with isoflurane and injected intravenously with ∼13 MBq of 76Br-L19-SIP (∼440 kBq/μg) in 150 μL via the femoral vein. The imaging sessions were performed at 5 h (one 15-min frame), 24 h, and 48 h after injection (one 30-min frame) using the microPET Focus (Siemens Medical Solutions USA, Inc.). At 24 and 48 h after injection, the mice underwent also microCT imaging (MicroCAT II; CTI-Imtek). microPET images (corrected for attenuation, scatter, normalization, and camera dead-time) and microCT images were coregistered using a landmark registration technique (by using fiducial markers directly attached to the animal bed) and AMIRA image display software (AMIRA; TGS Inc.). Data analysis of microPET images was performed using the manufacturer's software (ASIPRO; Siemens Medical Solutions). Data were calculated in terms of the standardized uptake values (SUVs) in 3-dimensional (3D) regions of interest (ROIs) using the following equation:
In Vivo Metabolism Studies
Normal female BALB/c mice (n = 3 per time point) weighing ∼20 g were anesthetized with isoflurane and injected intravenously with ∼1.8 MBq of 76Br-L19-SIP (∼185 kBq/μg) in 150 μL via the tail vein. The mice where anesthetized before sacrifice at each time point (2, 5, and 24 h after injection). Blood and urine samples were collected. Serum (∼150 μL) was separated from blood in Microtainer tubes (Becton, Dickinson and Co.). The residual immunoreactivity in serum was determined; then serum and urine samples were filtered through 0.45 μm and analyzed by radio-FPLC.
RESULTS
L19-SIP Radiolabeling with 76Br
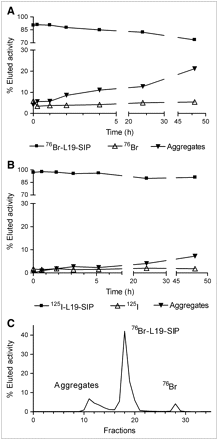
L19-SIP was reacted with different amounts of 76Br (∼25 MBq (∼0.67 mCi) or ∼90 MBq (∼2.43 mCi) per 100 μg antibody) for biodistribution and imaging studies. When reacting the compound with low amounts of 76Br (<37 MBq), 82% ± 2% radiolabeling yield was achieved in 80 min (n = 4) (Fig. 1). When using higher amounts of 76Br (>37 MBq), the 76Br solution recovered from the target processing was blown down to dryness and then reconstituted in the labeling buffer immediately before the reaction to avoid radiolytic effects. Under these conditions, 55% radiochemical yield was achieved in 80 min (n = 2). A complete separation between the radiolabeled product and the residual free 76Br-bromide could not be achieved with the NAP-5 chromatographic method. However, the 76Br-L19-SIP used for animal experiments had >90% RCP, as confirmed by radio-FPLC (Fig. 2B). The immunoreactivity of 76Br-L19-SIP was 80% ± 2% (n = 5), as measured by affinity chromatography.
Effect of reaction time on labeling yield of BPO-catalyzed L19-SIP 76Br-bromination at 0°C (<37 MBq 76Br used in the reactions; n = 3). Data are expressed as mean ± SD.
Representative FPLC chromatograms of L19-SIP (A) and NAP-5–purified 76Br-L19-SIP (76Br-L19-SIP) (B): Retention time [Rt] = 16.9–17.1 min; 76Br-bromide: Rt = 27.8 min). mAu = milliabsorbance units.
The in vitro stability of 76Br-L19-SIP in mouse serum at 37°C was investigated by radio-FPLC analysis. A sample with 91% RCP was used for this experiment. In 48 h, no additional 76Br-bromide was detected on the radiochromatogram (Fig. 3A). However, the formation of high-molecular-weight impurities was observed (21% of total activity in 48 h; Fig. 3C). In a similar experiment, the formation of high-molecular-weight impurities was observed also when incubating 125I-L19-SIP in mouse serum, but to a lesser extent compared with the 76Br-labeled analog (up to 7% of total activity in 48 h, Fig. 3B). Similarly, no radiolabel release from 125I-L19-SIP was observed in vitro.
In vitro stability of 76Br-L19-SIP (A) and 125I-L19-SIP (B) and radiochromatogram of 76Br-L19-SIP after 48-h incubation in mouse serum at 37°C (C).
Biodistribution Studies
F9 tumor-bearing mice were injected with a low dose of 76Br-L19-SIP (∼1.3 MBq/mouse corresponding to ∼0.35 mg antibody per kg mouse weight). The obtained biodistribution data (Table 1) showed a high and persistent activity accumulation in the F9 tumors (18.1 ± 7.6, 9.3 ± 3.5, and 14.3 ± 1.6 %ID/g at 5, 24, and 48 h, respectively) and in other ED-B fibronectin-expressing organs (ovaries and uterus). A significant amount of radioactivity was also observed in blood (22.4 ± 3.7, 7.6 ± 1.8, and 8.1 ± 1.7 %ID/g at 5, 24, and 48 h after injection, respectively), in blood-rich organs (such as lung and heart, which were not perfused before counting), and in other nontarget organs. Slow clearance was observed in many of the nontarget organs (except for muscle and bone) up to 48 h after injection as a consequence of slow radioactivity elimination through the urinary tract (4.7 ± 0.9 %ID at 48 h after injection in urine).
Biodistribution of 76Br-L19-SIP in F9 Tumor-Bearing 129/sv Mice at Various Time Points
Imaging Studies
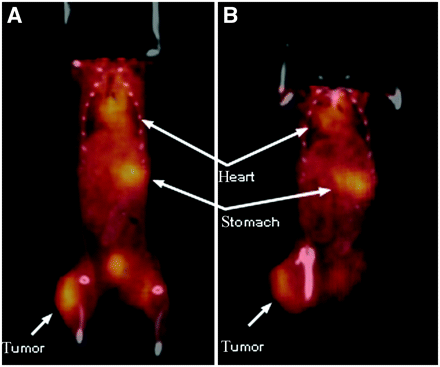
Four tumor-bearing mice were injected with a higher dose of 76Br-L19-SIP (∼13 MBq/mouse corresponding to ∼1.4 mg antibody per kg mouse weight). microPET images were collected at 5, 24, and 48 h after injection and coregistered with microCT at the last 2 time points. Specific accumulation of radioactivity in the ED-B fibronectin-expressing tumors was clearly visible at each considered time point (Figs. 4 and 5). As predicted from the biodistribution studies, the microPET images acquired at 5 h after injection exhibited a high background activity, mainly in the abdominal area. The heart and the aorta of the mice were clearly delineated at 5 h after injection due to the high amount of radioactivity in the blood (Figs. 4A and 4D). At this early time point, also the kidneys were visible. At 24 h after injection, the background activity was visibly lower compared with the earlier time point (Figs. 4B and 4E), whereas the stomach of both mice became detectable. At 48 h after injection, some background activity was still present and the most visible organs were the tumors, stomachs, and bladders (Figs. 4C and 4F). Semiquantitative analysis of microPET images (Table 2) confirmed the results of the biodistribution experiment. The tumor SUV was high and persistent at each considered time point (2.4 ± 0.5 at 5 h, 2.7 ± 0.1 at 24 h, and 2.4 ± 0.2 at 48 h after injection) and approximately 4- to 5-fold higher compared with that of the muscle. The stomach showed no specific accumulation of radioactivity over the surrounding tissues at 5 h after injection but became visible at later time points (SUV: 1.9 ± 0.0 at both 24 and 48 h after injection).
Coronal microPET projection images of 2 F9 tumor-bearing mice at 5 h (A and D), 24 h (B and E), and 48 h (C and F) after injection. Imaging intensity was decay-corrected and scaled by maximal/minimal (Max/Min) frame. a = aorta; t = tumor; h = heart; k = kidney; is = injection site; s = stomach; b = bladder. Red arrows indicate the fiducial markers used for microPET/microCT coregistration.
Coregistered microPET and microCT coronal images of one F9 tumor-bearing mouse injected with 76Br-L19-SIP at 24 h (A) and 48 h (B) after injection. In B, animal was slightly offset from full supine position because of tumor in right hindlimb. Imaging intensity was decay-corrected and scaled by maximal/minimal frame.
Comparative Organ-by-Organ SUVs for 76Br-L19-SIP from Quantitation of microPET Images in F9 Tumor-Bearing Mice (n = 4)
Metabolism Studies
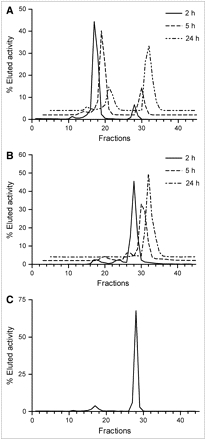
Normal mice were used for this study (n = 3 per time point). The animals were injected with ∼0.2 mg of 76Br-L19-SIP per kg body weight (∼1.8 MBq/mouse; RCP > 95%; 84% immunoreactivity). Serum and urine samples were collected and analyzed by radio-FPLC at selected time points. In serum, the amount of residual intact antibody was 86.1% ± 1.7% at 2 h after injection, 73.5% ± 0.5% at 5 h after injection, and 24.7% ± 0.9% at 24 h after injection (Fig. 6A) and the residual immunoreactivity of 76Br-L19-SIP was 65%, 66%, and 21% as determined by affinity chromatography. Small amounts of intact antibody were also detected in urine (Fig. 6B; 7.7% ± 0.1%, 5.8% ± 0.6%, and 0.8% ± 0.3% of total eluted activity at 2, 5, and 24 h after injection, respectively). However, the majority of the excreted activity was due to 76Br-bromide.
In vivo metabolism: radiochromatograms of plasma (A) and urine (B) of normal mice injected with 76Br-L19-SIP and radiochromatogram of plasma (C) withdrawn from a F9 tumor-bearing mouse microPET imaging (48 h after injection).
DISCUSSION
Molecular imaging of tumor neovasculature is an important feature for and early assessment of response to antiangiogenic cancer therapy. To date, the application of PET to angiogenesis imaging has been explored mostly by using radiolabeled RGD peptides targeting αvβ3 integrins. Small monomeric and dimeric RGD derivatives labeled with 64Cu (11) and 18F (8,12,14) showed a moderate accumulation in solid tumors but washed out within few hours after tracer administration. A higher and more persistent uptake was observed with a tetrameric RGD peptide, reasonably due to polyvalency and size (13). In all of these studies, however, variable tracer amounts were retained in nontarget organs such as lung, liver, kidney, and intestine. Despite this, a good correlation between αvβ3 expression and tumor uptake was observed in humans (10), and a variety of cancers was successfully imaged in a small clinical trial (9). High and prolonged uptake in angiogenic tumor vessels was obtained also by using small proteins such as the 64Cu-labeled VEGF121 (5) and the124I-labeled VG76e (7), targeting the VEGFR. Consequently, a radioiodinated humanized derivative of VGEF121 was tested as an antivascular therapeutic agent in a phase I clinical trial (6). Nonetheless, these compounds also showed nonspecific accumulation in nontarget organs such as liver, kidney, and lung.
The oncofetal domain (ED-B) of fibronectin has proven to be another powerful target on solid tumor neovasculature (15–17,34,35), and an antibody with subnanomolar affinity for ED-B fibronectin (L19) has been produced (18). Recently, radioimmunotherapy and SPECT with L19 derivatives labeled with various radionuclides (131I, 123I, 99mTc) gave very promising results in different tumor models (20–24) and in a small clinical trial (19). For these reasons, we decided to explore targeting of ED-B fibronectin with a positron emitter–labeled L19-SIP for PET of tumor neovasculature.
Toward this aim, we have chosen 76Br, a radiohalogen decaying 55% by positron-emission (maximum β-energy, [βmax] = 3.941 MeV). Despite the emission of prompt γ-rays in the energy window of PET scanners (559 and 657 keV), imaging with 76Br is feasible (29,36), and the favorable half-life (t1/2 = 16.2 h) makes this radionuclide a good candidate for imaging antibodies in vivo (27–31). The radiobromination of L19-SIP was performed by means of an enzymatic method using BPO (28,37). This direct halogenation approach was chosen because of it is straightforward and because the BPO/H2O2 mixture was proven to be fast and effective in producing radiobrominated antibodies with retained immunoreactivity (27–29). In fact, L19-SIP was labeled at a low dose of 76Br (<37 MBq) in high yield and in a short time (Fig. 1). When using >37 MBq 76Br, however, no significant antibody labeling was observed. Instead, the presence of low-molecular-weight radioactive impurities in the reaction mixture was detected by radio-FPLC (data not shown), possibly as a consequence of radiolysis. To avoid this, an optimized protocol could be established by removing any solvent right after the target process. NAP-5 purification of the labeling mixture proved to be a suitable way for a timely and cost-effective separation of the product. The values of immunoreactivity obtained for 76Br-L19-SIP were slightly lower compared with those reported for 125/131I-labeled analogs (20,24). This was reasonably due to the presence of residual 76Br-bromide in the NAP-5–purified antibody sample (Fig. 2B). Even though the radiobrominated antibody was found to be stable over 48 h in vitro (Fig. 3A), the formation of some high-molecular-weight by-products occurred over time (Fig. 3C), suggesting the formation of aggregates. A similar behavior was observed for 125I-L19-SIP (Fig. 3B).
The tumor model used to evaluate 76Br-L19-SIP as an angiogenesis-targeting agent is a fast-growing murine teratocarcinoma (F9) expressing high levels of ED-B fibronectin (20,24,25). Our biodistribution data showed elevated and fast accumulation of 76Br-L19-SIP in F9 tumors (Table 1), similar to that of 125I- and 111In-labeled analogs (20,25) and higher than that of a smaller antibody fragment labeled with 99mTc (24) in the same tumor model. Furthermore, the tracer accumulation in the tumor was comparable to that reported for VEGFR targeting PET tracers (5,7) and higher than that observed with αvβ3 targeting peptides (8,11–14) in a variety of tumor models.
Specific targeting of ED-B fibronectin was confirmed also by the high 76Br-L19-SIP uptake in the mouse reproductive organs (uterus and ovaries), which physiologically express the ED-B of fibronectin (15,34). Unfortunately, long retention of radioactivity in blood and very slow renal excretion were also observed. As a consequence, the background activity in nontarget organs was higher than that reported for the 125I-labeled L19-SIP, and this resulted in lower target-to-nontarget ratios in all of the considered organs but thyroid (Table 1). Despite the slow blood kinetics and the high activity in nontarget organs, such as muscle and bone, high imaging contrast was achieved and the F9 tumors implanted in the hindlimb were clearly imaged by microPET at each considered time point (Figs. 4 and 5). The SUV data obtained from the semiquantitative analysis of microPET images (Table 2) confirmed high and persistent 76Br-L19-SIP uptake in the tumor, which was 4- to 5-fold higher compared with that in nontarget tissue (lumbar muscle) at each considered time point.
The presence of activity in nontarget organs and the persistent activity in blood suggest partial debromination of 76Br-L19-SIP in vivo. In fact, when mice were administered radiobromide, high radioactivity levels were still detected in blood after 48 h (28,38). Furthermore, autoradiographic studies in mice reported high radioactivity concentration in the gastric mucosa as early as 5 min after injection of 82Br-bromide (39). In our experiments, the slow activity accumulation in the stomach, which appeared on the microPET images only 24 h after injection (Fig. 4; Table 2), indicates that some debromination of 76Br-L19-SIP occurred within several hours of the tracer administration. The results of a metabolism study in normal mice confirmed slow in vivo debromination of 76Br-L19-SIP. In fact, low amounts of 76Br-bromide were detected in serum at 2 and 5 h after injection, whereas, at 24 h after injection, most of the activity eluted from the FPLC column was free 76Br (Fig. 6A). At the end of the microPET/microCT sessions, we performed the same test on the plasma of the mice used for imaging. At this time point, 2.2% ± 0.2% (n = 2) of the administered radioactivity was still present in blood but only a small fraction of this was due to intact antibody, whereas most of the radioactivity was free bromide (Fig. 6C). Partial in vivo dehalogenation was observed also for 125I-L19-SIP. In fact, the reported radioactivity uptake values in the stomach (23) and nonblocked thyroid (20,22) are comparable to those obtained when administering the mice free radioiodide (28,40). Low background uptake in nontarget organs was observed for 125I-L19-SIP (20,22,25), reasonably because free iodide clears rapidly from the blood and is taken up by the thyroid or is excreted through the kidneys (28,40).
Therefore, to improve the in vivo performances of the radiobrominated antibody, indirect labeling strategies that may lead to less metabolism or radiocatabolites with short in vivo half lives (26,30,31) will have to be considered.
CONCLUSION
In this study, we investigated the possible use of a 76Br-labeled L19 derivative to image the neovasculature of tumors undergoing angiogenesis with PET. Although the directly labeled antibody underwent partial in vivo dehalogenation, with consequent high-activity background and slow clearance, the targeting properties of 76Br-L19-SIP for ED-B fibronectin were confirmed. In fact, the F9 tumors exhibited a high and persistent radiotracer uptake and were clearly visualized by microPET at each considered time point. Further studies are needed to optimize the in vivo performances of 76Br-L19-SIP. However, this is an important step toward the imaging of angiogenesis biomarkers with PET.
Acknowledgments
The authors thank Richard Laforest, Nicole Fettig, Margaret Morris, Dawn Werner, Lori Strong, Jerrel Rutlin, Susan Adams, and Terry Sharp for excellent assistance in the biodistribution and imaging studies as well as Dieter Moosmayer and Guido Malawski for production and purification of the L19-SIP antibody fragment and Lucie Tang, Douglas J. Rowland, and Rajendra Singh for 76Br production. The production of 76Br at Washington University School of Medicine is supported by NCI grant R24 CA86307.
Footnotes
-
COPYRIGHT © 2007 by the Society of Nuclear Medicine, Inc.
References
- Received for publication February 2, 2007.
- Accepted for publication April 17, 2007.